1536
Human brain POCE MRS at 7T using a 13C birdcage coil and 8 transmit-receive 1H antennas with a 32-channel 1H receive array1Department of Radiology and Nuclear Medicine, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Radiology and Biomedical Imaging, Magnetic Resonance Research Center, Yale University School of Medicine, New Haven, CT, United States, 3Department of Biomedical Engineering, Yale University School of Medicine, New Haven, CT, United States, 4Department of Radiology, Radboud University Medical Center, Nijmegen, Netherlands
Synopsis
We have shown that it is feasible to perform POCE MRS in the human brain at 7T during a uniformly labeled [U-13C] glucose infusion in three healthy volunteers, using a 13C birdcage head coil combined with 8 transmit-receive 1H antennas with a 32-channel 1H receive array. STEAM-POCE and sLASER-POCE provided similar sensitivity for the [4-13C]-Glx-H4 signals. POCE examinations could be performed in two locations, i.e. the frontal and occipital lobe, in one session, allowing them to be easily merged with 1H MRI.
Glutamate (Glu) is the most abundant excitatory neurotransmitter with receptors throughout the brain. Abnormal glutamergic neurotransmission is associated with various neurological and psychiatric disorders, such as dementia, epilepsy, and schizophrenia.1
Dynamic 13C MRS with administration of 13C-labeled glucose is a powerful method for measuring neurotransmitter cycling and neuro-energetics.1 To improve sensitivity and localization, proton-observed, carbon-edited (POCE) MRS can be used in combination with 1H single-voxel localization (SVL) methods, such as semi-localization by adiabatic selective refocusing (sLASER) or stimulated echo acquisition mode (STEAM).1 Most studies that have been carried out in humans, used 1H and 13C surface coils, thus limited to one location in the brain, most often the occipital lobe.2-4 Importantly, a surface coil setup also does not allow state-of-the-art MRI as obtained with standard 1H head coils.
The aim of this study was to develop a setup for POCE MRS and 1H MRI at 7T. Here we show this setup for sLASER-POCE and STEAM-POCE MRS in the occipital and frontal lobe.
METHODS
Set-up
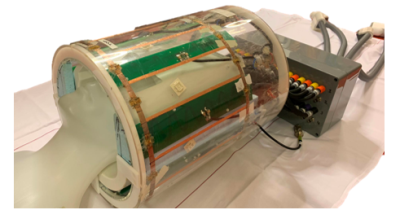
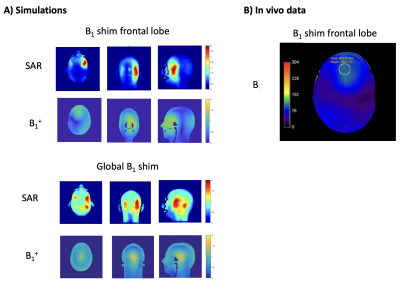
A 7T MR system (Philips, Best, NL) was used together with a 13C birdcage head coil combined with 8 transmit-receive 1H dipole antennas and a 32-channel 1H receive array (Nova Medical, Wilmington, USA; Figure 1). To ensure that the local specific absorption rate (SAR) limits were controlled for the 1H antennas when using either B1 focusing on the frontal lobe or global B1 shimming, electromagnetic simulations (Sim4Life, Zurich MedTech, ZMT) were done using the human model Duke from the virtual family.5
Sequences
POCE MRS was performed using sLASER or STEAM SVL (Figure 2). In both sequences, the POCE TE was set to 7.8 ms (tuned to the H4-C4 J-coupling constant of Glu and glutamine (Gln), together termed Glx) and a composite 13C 180° block pulse was used for 13C inversion, which was set to 32.95 ppm (in between the C4 resonances of Glu and Gln) and switched on and off every other acquisition. The sequences were tested in three healthy volunteers (male; 22-30 years) during a primed [U-13C]glucose infusion of 99% for 20 min and 60% for 100 min, with aimed plasma glucose levels of 6-10 mmol/l. sLASER-POCE was tested in one volunteer, STEAM-POCE in two, where in one session voxel placement was alternated between the frontal and occipital lobe. Acquisition parameters were as follows: sLASER-POCE: TE/TR=48/5000ms, NSA 2x32; STEAM-POCE: TE/TM/TR=7.8/30/3000ms, NSA 2x48. VAPOR water suppression (300 Hz) was used in both cases. B1+ and B0 shimming were performed on a manually drawn region of interest (ROI). No outer volume suppression was used.
Data analysis
Coil combination was performed using a generalized-least-squares algorithm, using the unsuppressed water signal to compute the coil sensitivity of each channel. Spectra were frequency and phase aligned and subtracted pairwise. The [4-13C]-Glx-H4 signals in the difference spectra were fitted in MATLAB (MathWorks, USA).
RESULTS
Electromagnetic simulations showed a peak local SAR of 1.94 versus 3.9 W/kg for an input power of 8 x 1 W, when driven in global head shim versus B1 focusing on the frontal lobe, providing a maximum B1+ of 1.18 mT (center) versus 1.26 mT (frontal) at this power level (Figure 3A). When driving the sLASER-POCE and STEAM-POCE to a maximum of 15 and 16 mT, respectively, while incorporating the added 3-5% of SAR by the 13C pulses, these scans remained well within the IEC SAR guidelines6 of 10 W/kg local and 3.2 W/kg global SAR at a TR of 5 and 3 s, respectively. The B1+ map measured in vivo after B1+ shimming in an ROI in the frontal lobe was in good agreement with the simulation (Figure 3B).
[4-13C]-Glx-H4 signals were detected in both sLASER-POCE and STEAM-POCE difference spectra (Figure 4A). Although SNR of the n-acetylaspartate (NAA) signal in the separate on/off spectra was ~50% lower for STEAM-POCE (taking into account differences in voxel size and acquisition times), the [4-13C]-Glx-H4 signals in the difference spectra had similar SNR. Time courses of 13C labeling of Glx-H4 showed similar kinetics for POCE-STEAM and POCE-sLASER (Figure 4B). [4-13C]-Glx-H4 could be detected in both the frontal and the occipital lobe within a single scan session (Figure 5).
DISCUSSION & CONCLUSION
We have shown that it is feasible to detect [4-13C]-Glx-H4 during a [U-13C]glucose infusion at 7T, using a 13C birdcage head coil combined with 8 transmit-receive 1H dipole antennas and a 32-channel 1H receive array, in two different locations in the brain, i.e. frontal and occipital, in one session. Moreover, we have shown that while STEAM voxel localization leads to ~50% signal loss of the uncoupled resonances in the unedited spectra compared to sLASER, SNR of the [4-13C]-Glx-H4 signals was similar in both the STEAM-POCE and sLASER-POCE difference spectra. This can be explained by the relatively larger signal loss for the strongly coupled Glx spin systems in sLASER due to the longer TE (48 vs 7.8 ms). On the other hand, chemical shift displacement errors are generally assumed to be smaller for sLASER, reducing the likelihood of artefacts from extracranial lipids. While a shoulder of [4-13C]-Gln-H4 could be observed, [4-13C]-Glu-H4 and [4-13C]-Gln-H4 signals were not clearly separated. Glu/Gln signal separation could be achieved by implementing selective POCE.7
Acknowledgements
No acknowledgement found.References
1. Rothman DL, de Graaf RA, Hyder F, et al. In vivo 13C and 1 H-[13 C] MRS studies of neuroenergetics and neurotransmitter cycling, applications to neurological and psychiatric disease and brain cancer. NMR Biomed. 2019 Oct;32(10):e4172
2. Chen W, Zhu X-H, Gruetter R, Seaquist ER, Adriany G, Ugurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using 1H-{13C} MRS and fMRI. Magn Reson Med. 2001;45:349–355
3. Pan JW, Stein DT, Telang F, et al. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn Reson Med. 2000;44:673–679
4. Rothman DL, Novotny EJ, Shulman GI, Howseman AM, Petroff OA, Mason G, Nixon T, Hanstock CC, Prichard JW, Shulman RG. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci U S A. 1992;89:9603–9606
5. Christ A, Kainz W, Hahn EG, Honegger K, et al. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010 Jan 21;55(2):N23-38
6. IEC 60601‐2‐33:2010, Medical electrical equipment—Part 2‐33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis, International Standard, Edition 3.0
7. De Feyter HM, Herzog RI, Steensma BR, et al. Selective proton-observed, carbon-edited (selPOCE) MRS method for measurement of glutamate and glutamine 13C-labeling in the human frontal cortex. Magn Reson Med. 2018 Jul;80(1):11-20Figures
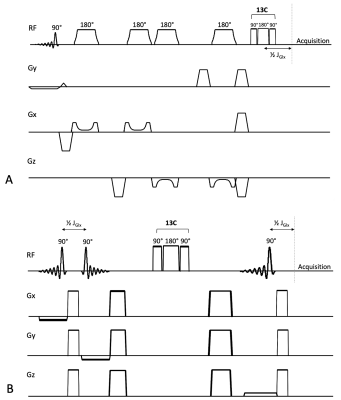
(A) sLASER-POCE and (B) STEAM-POCE pulse sequences.
STEAM-POCE difference spectra from volunteer 3 alternately scanned in the frontal (voxel size = 2.5 x 4.5 x 3 cm; left) and the occipital (voxel size = 3 x 4.5 x 3 cm; right) lobe, before and at two time points after the start of [U-13C]glucose infusion. Dashed black lines (at 2.13 and 2.55 ppm) and dashed red lines (at 2.23 and 2.66 ppm) indicate the resonance positions for the [4-13C]-Glu-H4 and [4-13C]-Gln-H4 signals, respectively.