1528
Optimization of combined spin- and gradient-echo EPI in fMRI via in- and through-plane acceleration1Barrow Neurological Institute, Phoenix, AZ, United States, 2Arizona State University, Tempe, AZ, United States
Synopsis
BOLD-fMRI is widely used to study brain activity but suffers from signal dropout near air-tissue interfaces and lack of sensitivity to microvasculature. A combined spin- and gradient-echo (SAGE) acquisition, previously implemented for perfusion MRI, may be able to reduce signal dropout and provide sensitivity to microvasculature for fMRI applications. SAGE-fMRI presents its own challenges, namely lack of full brain coverage and poor temporal and spatial resolution. These challenges can be overcome by combining in-plane and through-plane acceleration factors. This study aims to optimize the SAGE-fMRI protocol, balancing acceleration of acquisition with image quality.
Introduction
Blood oxygen level-dependent (BOLD) functional MRI (fMRI) is widely used to study both task-induced functional activation and resting-state functional connectivity. Acquiring fMRI with gradient-echo (GE) echo-planar imaging (EPI) presents several challenges, including signal dropout and image distortion near air-tissue interfaces (i.e., inferior frontal and temporal lobes)1,2. Additionally, while GE fMRI is sensitive to BOLD contrast, it is also dominated by large-vessel effects2, despite the metabolic exchange associated with BOLD taking place in microvascular capillary beds3. To improve acquisition in high-susceptibility regions and increase sensitivity to microvasculature, we propose use of a combined spin- and gradient-echo (SAGE)4,5 acquisition in fMRI; multi-GE imaging has been shown to improve BOLD sensitivity and signal-to-noise ratio (SNR) in regions with susceptibility effects1,6, and the addition of spin-echo (SE) imaging provides sensitivity to microvascular effects7. SAGE-fMRI presents its own challenges, namely inability to acquire full brain coverage and inadequate spatial and temporal resolution8. To acquire full brain coverage and improve spatial and temporal resolution, we aim to establish an optimal SAGE-fMRI protocol by testing combinations of multiband RF excitation (MB) and sensitivity encoding (SENSE) acceleration factors, balancing acceleration with image quality (i.e., SNR).Methods
Dynamic SAGE EPI data were acquired in healthy subjects (n=6, mean of 32.8 years old, 1 male) at 3T (Ingenia, Philips) with a dedicated 32-channel head coil. BOLD-fMRI data were acquired with a multi-echo, multi-contrast SAGE acquisition (2 GE, 2 asymmetric spin echo, and 1 SE (echo times (TE) vary with acceleration factors, Table 1)), as depicted in Figure 1. Additional acquisition parameters were as follows: repetition time (TR) = 3.0 s, acquisition matrix: 80 × 78, voxel size = 3 × 3 mm, slice thickness = 3 mm, in-plane field-of-view (FOV) = 240 mm, 40 volumes, acquisition time = 2 min. Echo times (TE) and number of axial slices acquired varied between acceleration factor combinations; the partial Fourier (PF) factor was held relatively constant9 (Table 1). For the sake of comparing SNR, factors outside of MB and SENSE were held constant; this negated some of the benefits of higher acceleration factors (e.g., full brain coverage, higher spatial resolution) that could be achieved and implemented in future work. The MB factor was varied at 1 (no MB factor), 2, 3, and 4, and the SENSE factor was varied at 2, 2.5, and 3. A total of 12 protocols, with 5 SAGE signals each, were thus compared.The signals from each SAGE acquisition underwent minimal pre-processing to correct for motion and distortion (AFNI10, FSL11). For each acquisition, the SAGE signals were fit voxel-wise to obtain R2* and R2, as follows5: $$S(TE) = \begin{cases}S_0^I\cdot exp[-TE\cdot R_2^* ] & 0<TE<\frac{TE_{SE}}{2}\\S_0^{II}\cdot exp[-TE_{SE}\cdot (R_2^*-R_2)]\cdot exp[-TE\cdot (2R_2-R_2^* )] & \frac{TE_{SE}}{2}<TE<TE_{SE}\end{cases}$$
where $$$S_0^I$$$ and $$$S_0^{II}$$$ represent the signal intensities following the excitation and refocusing pulses, respectively, and TESE is the SE TE. The SAGE-based T2* (= 1/R2*) and T2 (= 1/R2) maps were compared to standard Philips T2* and T2 maps acquired using multi-GE and multi-SE methods, respectively.
Temporal signal-to-noise ratio (tSNR) was calculated for all acquisitions for TE2 and TE5 (representing a standard GE and SE acquisition, respectively). Voxel-wise tSNR was calculated using the mean signal intensity over time ($$$\overline{S}$$$) divided by the standard deviation of the noise over time ($$$\sigma_{noise}$$$): $$tSNR= \frac{\overline{S}}{\sigma_{noise}}$$
A one-way ANOVA model was implemented to compare mean group tSNR across acceleration factor combinations (FDR corrected q<0.05) and mean group T2* and T2 values across acceleration factor combinations; Tukey’s HSD was used for post-hoc analysis.
Results
Using a combination of MB, SENSE, and PF, SAGE TEs could be obtained with TE1 less than 10 ms and TE5 less than 120 ms. MB factors of 2, 3, and 4 yielded full brain coverage, while MB-1 did not. Qualitative analysis of TE2 and TE5 revealed loss of signal and image quality in MB4 across SENSE factors (Figure 1). Quantitative assessment of tSNR via pairwise comparisons revealed no significant differences in tSNR between any of the MB2 and MB3 combinations across SENSE factors (Figure 1). Qualitative analysis of T2* and T2 SAGE maps showed that MB2 and MB3 across SENSE factors exhibited good image quality and that MB4 across SENSE factors mimicked the distortion seen in TE2 and TE5 individually (Figure 2). Quantitative assessment of T2* and T2 SAGE maps revealed no significant differences between any of the MB2 and MB3 combinations across SENSE factors (Figure 2).Discussion
The combination of in-plane acceleration with SENSE and through-plane acceleration with MB factors allowed for full brain coverage and improvement in spatial and temporal resolution, while avoiding significant loss in image quality and tSNR. Based on our analyses, we recommend the MB2-SENSE2.5 combination in SAGE-fMRI due to its minimal loss in tSNR and sufficient improvement in spatial and temporal resolution.Conclusion
SAGE-fMRI, leveraging MB and SENSE acceleration factors, allows for full brain acquisition and improved temporal and spatial resolution without significant loss in tSNR. Future directions include application of both task-based and resting-state SAGE-fMRI in clinical populations.Acknowledgements
We acknowledge Philips and the sources of funding for this work, the Arizona Alzheimer's Consortium and the Barrow Neurological Foundation.References
1. Poser BA, Versluis MJ, Hoogduin JM, Norris DG. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med. 2006;55(6):1227-1235.
2. Glover GH, Lemieux SK, Drangova M, Pauly JM. Decomposition of inflow and blood oxygen level-dependent (BOLD) effects with dual-echo spiral gradient-recalled echo (GRE) fMRI. Magn Reson Med. 1996;35(3):299-308.
3. Sanders J, Orrison W. Functional magnetic resonance imaging. Functional brain imaging. St Louis Mosby Year B. 1995:250-253.
4. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30-40.
5. Stokes AM, Skinner JT, Quarles CC. Assessment of a combined spin- and gradient-echo (SAGE) DSC-MRI method for preclinical neuroimaging. Magn Reson Imaging. 2014;32(10):1181-1190.
6. Halai AD, Parkes LM, Welbourne SR. Dual-echo fMRI can detect activations in inferior temporal lobe during intelligible speech comprehension. Neuroimage. 2015;122:214-221.
7. Norris DG, Zysset S, Mildner T, Wiggins CJ. An investigation of the value of spin-echo-based fMRI using a stroop color-word matching task and EPI at 3 T. Neuroimage. 2002;15(3):719-726.
8. Bergamino M, Steffes L, Gonzales A, et al. Optimization of vascular and functional sensitivity using multi-contrast, multi-echo SAGE-EPI for fMRI. Proc 28th Annu Meet ISMRM Sydney, Aust. 2020:5428.
9. Skinner JT, Robison RK, Elder CP, Newton AT, Damon BM, Quarles CC. Evaluation of a multiple spin- and gradient-echo (SAGE) EPI acquisition with SENSE acceleration: Applications for perfusion imaging in and outside the brain. Magn Reson Imaging. 2014;32(10):1171-1180.
10. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173.
11. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL 1. Neuroimage. 2012;62(2):782-790.
Figures
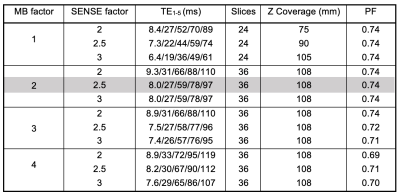
Table 1. Acquisition parameters across MB and SENSE factors. Optimal sequence in highlighted row. While all MB factors > 2 permit full brain coverage (>120 mm), the number of slices was limited to 36 for comparisons across acceleration factors. Wherever possible, all factors outside of MB and SENSE were held constant for consistency.
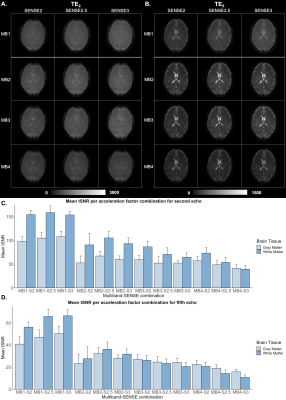
Figure 1. Single-subject qualitative assessment of A.) TE2 (GE) and B.) TE5 (SE) across acceleration factor combinations. Group mean tSNR for C.) TE2 and D.) TE5 in gray and white matter across acceleration factor combinations.
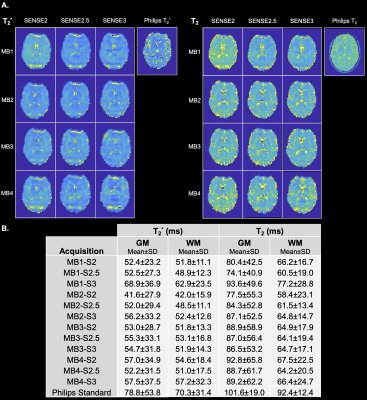
Figure 2. A.) Single-subject qualitative assessment of T2* and T2 across acceleration factor combinations. Standard Philips T2* and T2 maps are also shown for comparison. B.) Group-level mean and standard deviation T2* and T2 in gray and white matter across acceleration factor combinations and the Philips standard acquisitions.