1525
T2-weighted Free-breathing 3D Abdominal Imaging Using Magnetization Prepared SPGR1Radiology, Stanford University, Stanford, CA, United States
Synopsis
T2-weighted imaging is conventionally obtained using multi-slice 2D or 3D fast spin-echo (FSE) sequences. However, FSE suffers from severe specific absorption rate (SAR) issue and motion artifact particularly in the abdomen. A potential approach to address these challenges is using T2-prepared 3D SPGR sequence with a non-Cartesian trajectory such as cones. In this study, we proposed a T2-prepared 3D-cones sequence to achieve T2-weighted abdominal imaging with free breathing. The feasibility of the technique was tested on both healthy subjects and patients.
Introduction
T2-weighted imaging is important in clinical practice and is typically obtained with multi-slice 2D or 3D fast spin-echo (FSE) sequences. However, these have high specific absorption rate (SAR) and suffer from motion artifacts particularly in abdominopelvic applications1,2. To address these challenges, many approaches have been proposed such as variable refocusing flip-angle to decrease the SAR and PROPELLER3 to address respiratory motion. An alternative approach is using T2-prepared 3D SPGR sequences, as demonstrated in several studies4–6. To further address the motion issue, a non-cartesian trajectory can be incorporated. One such trajectory is the 3D-cones, which can provide ultra-short time of echo (TE) and exhibits good motion properties that can be quite useful in abdominal imaging7,8. Therefore, the purpose of this study is to propose a T2-prepared 3D-cones sequence and assess its feasibility to provide free-breathing abdominal imaging on both healthy subjects and patients.Methods
T2-prepared 3D-Cones:As shown in Figure 1, a T2 preparation module was designed with 4 Malcolm-Levitt (MVTL) refocusing pulses, which is expected to be more robust to B1 inhomogeneity6. After the -90º tip-up pulse, a large spoiling gradient was played to destroy any residual transverse magnetization. Following the T2 preparation module, N acquisition blocks of 3D cones were added (N=20 in this study). Then, a wait time is added to allow longitudinal magnetization recovery to provide adequate T2 contrast. Although the MVTL-4 is designed to be robust to B1 inhomogeneity, the image can still exhibit banding artifacts when the B1 inhomogeneity is excessive. Thus, a pair of crusher gradients were added to each of the 180º refocusing pulses to remove the banding artifact. Fat suppression was achieved using a 2.3ms hard RF pulse9.
Data Acquisition:
A T2-prepared 3D-cones sequence (Figure 1) was implemented on a 3T GE MR750/Premier scanner. With IRB approval, abdominal MR images were acquired from four healthy human subjects and two pediatric patients with free-breathing. The key sequence parameters were: T2 preparation time=75ms, TR=1000ms, time of each acquisition block=8.0ms, FOV=420×420×180 mm3, matrix size = 320×320×120. The total acquisition time was about 5 minutes. For one subject the data was acquired using shorter readout (acquisition block of 6.5ms) that took ~10 minutes. If banding artifact presents, the acquisition will be repeated by adding crusher gradients. On patients, multi-slice T2-weighted 2D FSE and contrast-enhanced images using the conventional 3D-cones were acquired as a comparison.
Image Reconstruction:
The basic image reconstruction was performed offline using a custom Python program based on the gridding algorithm (non-uniform FFT) provided in BART10. Zero-padding was used to double the matrix size in all datasets. To reduce the off-resonance artifacts, a pre-trained deep neural network was also applied to the reconstructed images from the previous step11.
Results
On subjects that experienced excessive B1 inhomogeneity, banding artifacts were introduced into the image (Figure 2A). The artifacts were removed by adding crusher gradients in the T2 preparation pulses (Figure 2B). Figure 3A showed the blurred image arising from off-resonance, which can be mitigated by decreasing the readout (Figure 3B), but at the expense of doubling the acquisition time. The image corrected using deep learning neural network has also been improved compared with gridding reconstruction as shown in Figure 4. More importantly, the T2-prepared 3D-cones provides motion-free images with bright vessels clearly seen in the liver (Figure 5B) as in contrast-enhanced T1-weighted 3D-cones (Figure 5C), whereas the conventional T2-weighted 2D FSE was corrupted by motion artifact (Figure 5A).Discussion and Conclusion
Free-breathing abdominal imaging with T2-contrast was successfully performed on both healthy subjects and patients using a T2-prepared 3D-cones sequence. The T2-prepared techniques have previously been used in cardiac imaging4,12,13. In this study, motion-free images were acquired with T2-contrast in abdomen using 3D SPGR sequence for the first time. Although in most cases banding artifacts were not observed, the images from one subject did show banding artifacts probably due to excessive B1 inhomogeneity14. Adding crusher gradients can effectively reduce the banding artifact as demonstrated in this study, which although requires further B1 correction. Another approach to mitigate the problem is using adiabatic RF pulses5,14. Stretching the readout length of 3D-cones can result in blurred images due to off-resonance, which can be partially resolved by using deep learning neural network reconstruction without adding extra acquisition time. Our next step will be further shortening the acquisition time and improving the image quality to increase its clinical acceptance.Acknowledgements
This work was supported in part by NIH R01EB009690, NIH U01EB029427, and GE Healthcare.References
1. Takayama, Y. et al. Three-dimensional T2-weighted imaging for liver MRI: Clinical values of tissue-specific variable refocusing flip-angle turbo spin echo imaging: 3D T2-Weighted Imaging for Liver MRI. J. Magn. Reson. Imaging 41, 339–346 (2015).
2. Ream, J. M. and Rosenkrantz, A. B. Advances in T1-Weighted and T2-Weighted Imaging in the Abdomen and Pelvis. Radiol. Clin. North Am. 53, 583–598 (2015).
3. Pipe, J. G. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. 42, 963–969 (1999).
4. Zhu, J. et al. T2-prepared segmented 3D-gradient-echo for fast T2-weighted high-resolution three-dimensional imaging of the carotid artery wall at 3T: a feasibility study. Biomed. Eng. OnLine 15, 165 (2016).
5. Klupp, E. et al. B1-insensitive T2 mapping of healthy thigh muscles using a T2-prepared 3D TSE sequence. PLOS ONE 12, e0171337 (2017).
6. Brittain, J. H. et al. Coronary Angiography with Magnetization-PreparedT2 Contrast. Magn. Reson. Med. 33, 689–696 (1995).
7. Gurney, P. T., Hargreaves, B. A. & Nishimura, D. G. Design and analysis of a practical 3D cones trajectory. Magn. Reson. Med. 55, 575–582 (2006).
8. Chen, B. et al. Three-dimensional ultrashort echo time cones (3D UTE-Cones) magnetic resonance imaging of entheses and tendons. Magn. Reson. Imaging 49, 4–9 (2018).
9. Norbeck, O. et al. Optimizing 3D EPI for rapid T 1 ‐weighted imaging. Magn. Reson. Med. 84, 1441–1455 (2020).
10. Uecker, Martin et al. mrirecon/bart: version 0.7.00. (Zenodo, 2021). doi:10.5281/ZENODO.592960.
Figures
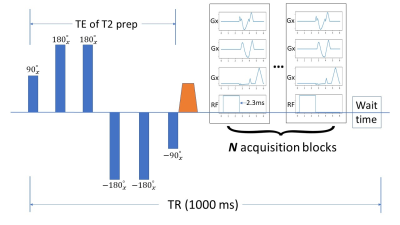
Figure 1: A sequence diagram used in this study with T2 preparation module in a 3D-cones sequence. Four MLVT pulses were designed to be more robust to B1 inhomogeneity. A large spoiling gradient is played after the tipup pulse to destroy the residual transverse magnetization. Followed by the T2 preparation module is a set of 3D-cones acquisition blocks (N = 20 in this study). For each TR, a wait time is also required to allow for the longitudinal magnetization to recover. A 2.3 ms hard RF pulse is used to achieve fat suppression.
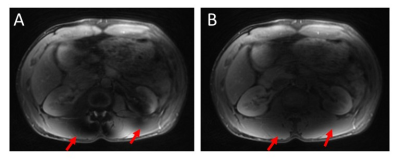
Figure 2: Illustration of the banding artifact resulting from B1 inhomogeneity (A, indicated by the red arrows). The artifacts can be reduced by adding a pair of crushers in the T2 preparation pulses (B).
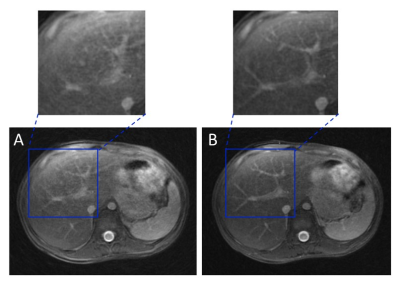
Figure 3: Illustration of the blurred image due to off-resonance using longer cones readout (8ms, A). Decreasing the readout can provide better image quality (6.5ms, B), however, at the expense of doubling the acquisition time.
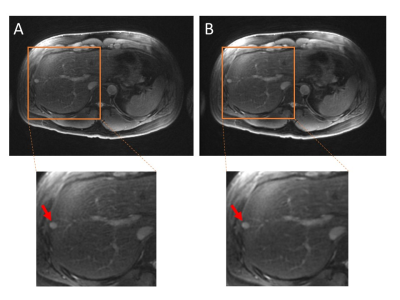
Figure 4: Liver images acquired from a healthy subject (male, 29 yo) using gridding reconstruction (A) and neural network off-resonance correction (B), respectively. A focal lesion can be clearly identified from both images as indicated by the red arrow. Compared with the gridding reconstruction alone, neural network off-resonance correction provides enhanced image quality.
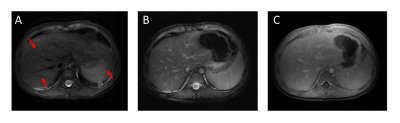
Figure 5: Liver images acquired from a pediatric patient (two years-old) using T2-weighted 2D FSE (A), T2-prepared 3D-cones (B) and contrast-enhanced T1-weighted 3D-cones (C), respectively. The 2D acquisition suffers motion artifacts (indicated by the red arrows), whereas 3D-cones sequence is robust to motion. The T2 contrast provided by T2-prepared 3D-cones makes the vessels bright, which is comparable to the contrast-enhanced images.