1512
Microbleed detection in autopsied brains from community-based older adults using microbleed synthesis and deep learning
Grant Nikseresht1, Ashish A. Tamhane2, Carles Javierre-Petit3, Arnold M. Evia2, David A. Bennett2, Julie A. Schneider2, Gady Agam1, and Konstantinos Arfanakis2,3
1Department of Computer Science, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States
1Department of Computer Science, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States
Synopsis
Automated cerebral microbleed (CMB) detection on ex-vivo MRI is key to enabling MRI-pathology studies in large community-based cohorts where manual CMB annotation is time consuming and prone to error. The aim of this study is to develop a CMB detection algorithm to aid in the quantization and localization of CMBs on ex-vivo T2*-weighted gradient echo MRI in community-based cohorts. A CMB synthesis algorithm is proposed and synthetic CMBs are used to train a neural network for CMB detection. A model trained with both synthetic and real data is shown to outperform models trained on synthetic or real data alone.
Introduction
Identification of cerebral microbleeds (CMBs) on ex-vivo MRI is important to MRI-pathology studies of cerebral small vessel disease1-3. The aim of this study was to develop an algorithm for the detection of CMBs on ex-vivo MRI of autopsied brains from community-based older adults where the average number of CMBs per person is very low (i.e. real training data is scarce) and mimics are highly prevalent (e.g. air bubbles attached to the surface of the brain) (Fig. 1). A customizable CMB synthesis algorithm is proposed for generating CMBs and CMB mimics which enables the training of a deep learning CMB detector even with extremely limited amounts of real data.Methods
Cerebral hemispheres from 80 autopsied participants of two cohort studies of aging, the Rush Memory and Aging Project4 and Religious Orders Study5, were included in this work. Half of the participants were used for dataset synthesis and detector training, and half were used as a holdout set for evaluation. Ex-vivo T2*-weighted images at 4 gradient echoes and with a voxel size of 1x1x1 mm3 were used after N4 bias correction. Manual annotation of CMBs in these images was done by an experienced rater blinded to all clinical and pathological information.Synthesis of CMBs was done by sampling patches of healthy tissue from the 40 training participants and placing round hypointensities at the center (Fig. 2). Background patches were sampled to avoid pre-existing CMBs and the edges of tissue. An 11x11x11 voxel Gaussian filter with values in the range (0, 1] was multiplied with a map of estimated T2* values in order to produce a synthetic hypointensity at the center of the patch (Fig. 3). The T2*-weighted signals in the 4 echoes were then regenerated using the T2* decay equation to produce a synthetic CMB. Random size, shift, and rotation were also applied to the Gaussian filter. CMB mimics were also simulated using this same method. Structures resembling blood vessels were synthesized by increasing the standard deviation in 1 or 2 of the 3 dimensions in the Gaussian filter. Air bubbles were synthesized by placing synthetic CMBs onto background patches centered outside of tissue.
A support vector machine (SVM) based candidate selection classifier was trained on a training set of 171 real CMB voxels and 27,408 non-CMB voxels where each sample was made up of 27 point-wise image features. Training was done with a high class weight on positive examples to produce a high sensitivity, low precision classifier. Patches of voxel size 16x16x16x10 with 4 signal channels and 6 PCA channels were then used to train a 3D convolutional neural network classifier based on ResNet18 to detect patches that are centered on CMBs6. A balanced dataset of 80,000 synthetic CMB patches and 80,000 non-CMB patches made up of synthetic CMB mimics, real CMB mimics, and background was used for training. 1,998 patches from 74 real CMBs were injected into mini-batches during training in place of synthetic CMBs. The deep learning model was evaluated on the holdout subjects in a 3x3x3 region around voxels selected by the candidate selector. A 3x3x3 identity matrix was convolved with the final probability map to identify peak regional detections. Non-maximum suppression was applied to produce final detections.
Results
Participants in the present community-based cohort average fewer than 2 CMBs per scan, while CMB mimics on ex-vivo scans greatly outnumber true CMBs due to partial volume effects caused by air bubbles that are not present in-vivo. A combination of synthetic CMBs with four real CMBs per training batch achieved the highest average precision (0.2522) compared to synthetic only (0.1750) and real only (0.1494) training sets. The model trained on both synthetic and real data achieved sensitivity of 81% at 15 false positives per subject, compared to 63% when training was based only on synthetic data, and 52% when training only on real data (Fig. 4).Discussion
CMB synthesis provided an efficient approach to substantially improve classifier performance by increasing the variability of both CMBs and backgrounds in training datasets. Recent work has also demonstrated the viability of synthetic CMBs for training deep neural networks7, but the present work is the first to consider synthesis of CMBs in the setting of ex-vivo MRI of autopsied brains from community-based individuals, as well as include synthesis of CMB mimics. While automated CMB detection algorithms have been shown to achieve strong performance in-vivo, they have access to participants from clinical settings with large numbers of CMBs for training and evaluation and fewer CMB mimics8-11. The CMB synthesis and detection algorithm presented in this work is able to achieve partial automation despite the challenges present in the ex-vivo setting and can aid in manual annotation of CMBs in future studies.Conclusion
This work shows that training a deep learning algorithm for detecting CMBs on ex-vivo scans of participants from a community-based cohort is made feasible by using synthetic CMB training data. This algorithm will enable the semi-automatic quantification and localization of ex-vivo CMBs in future MRI-pathology studies and help further investigation into the neuropathological correlates of CMBs and the potential of CMBs as a feature in classifiers of neuropathology.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2-UH3NS100599National Institute of Neurological Disorders and Stroke (NINDS) UF1NS100599
National Institute on Aging (NIA) R01AG064233
National Institute on Aging (NIA) R01AG067482
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) R01AG015819
National Institute on Aging (NIA) P30AG010161
National Institute on Aging (NIA) P30AG072975
References
1. Martinez-Ramirez S, Romero JR, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11(12):1480-1488.2. Guidoux C, Hauw JJ, Klein IF, et al. Amyloid Angiopathy in Brain Hemorrhage: A Postmortem Neuropathological-Magnetic Resonance Imaging Study. Cerebrovasc Dis. 2018;45(3-4):124-131.
3. Pasi M, Pongpitakmetha T, Charidimou A, et al. Cerebellar Microbleed Distribution Patterns and Cerebral Amyloid Angiopathy [published correction appears in Stroke. 2019 Aug;50(8):e240]. Stroke. 2019;50(7):1727-1733.
4. A. Bennett D, A. Schneider J, S. Buchman A, L. Barnes L, A. Boyle P, S. Wilson R.
Overview and Findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663.
5. A. Bennett D, A. Schneider J, Arvanitakis Z, S. Wilson R. Overview and Findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628-645.
6. Szegedy C., Liu W., Jia Y., Sermanet P., Reed S., Anguelov D. Proceedings of the IEEE conference on computer vision and pattern recognition. 2015. Going deeper with convolutions; pp. 1–9.
7. Momeni S, Fazlollahi A, Yates P, et al. Synthetic microbleeds generation for classifier training without ground truth. Comput Methods Programs Biomed. 2021;207:106127. doi:10.1016/j.cmpb.2021.106127
8. Liu S, Utriainen D, Chai C, et al. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage. 2019;198:271-282. doi:10.1016/j.neuroimage.2019.05.046
9. Chen Y, Villanueva-Meyer JE, Morrison MA, Lupo JM. Toward Automatic Detection of Radiation-Induced Cerebral Microbleeds Using a 3D Deep Residual Network [published correction appears in J Digit Imaging. 2019 Feb 8;:]. J Digit Imaging. 2019;32(5):766-772. doi:10.1007/s10278-018-0146-z
10. Morrison MA, Payabvash S, Chen Y, et al. A user-guided tool for semi-automated cerebral microbleed detection and volume segmentation: Evaluating vascular injury and data labelling for machine learning. Neuroimage Clin. 2018;20:498-505. Published 2018 Aug 4. doi:10.1016/j.nicl.2018.08.002
11. Qi Dou, Hao Chen, Lequan Yu, et al. Automatic Detection of Cerebral Microbleeds From MR Images via 3D Convolutional Neural Networks. IEEE Trans Med Imaging. 2016;35(5):1182-1195. doi:10.1109/TMI.2016.2528129
Figures
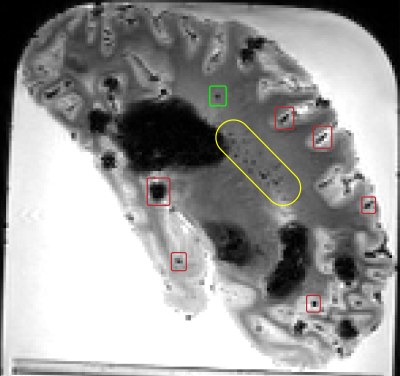
Figure 1. Examples of a real CMB (green box), air bubbles (red boxes), and a region with numerous blood vessels (yellow) on a sagittal ex-vivo T2*-weighted image.
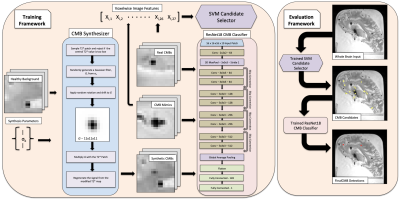
Figure 2. An overview of the CMB synthesis and detection framework.
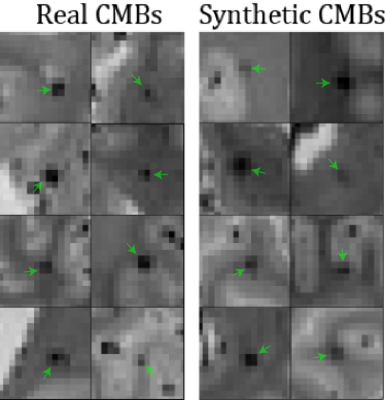
Figure 3. Examples of slices from real CMB patches (left) compared to synthetic CMB patches (right). Green arrows indicate the CMB hypointensity at the center of the patch.
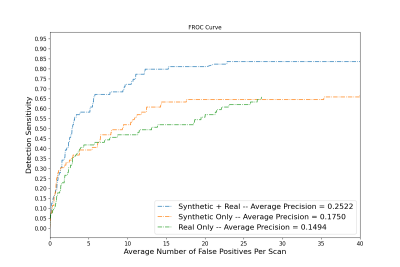
Figure 4. Free receiver operating characteristic (FROC) curve showing detector performance in terms of average number of false positives per scan for three models trained on synthetic only, real only, and synthetic + real training datasets.
DOI: https://doi.org/10.58530/2022/1512