1505
A Hybrid IVIM-DTI Model with Optimized Acquisition Time1Department of Medical Physics and Biomedical Engineering, Tehran University of Medical Sciences, Tehran, Iran (Islamic Republic of), 2Department of Medical Physics and Biomedical Engineering, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran (Islamic Republic of), 3Quantitative MR Imaging and Spectroscopy Group, Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran (Islamic Republic of)
Synopsis
IVIM-DTI imaging is capable of revealing both structural and functional maps. We conduct this study to address the long acquisition time of IVIM-DTI imaging and the inaccuracy of estimates of the model’s outputs. Diffusion-weighted images were acquired at 11 b-values and 64 orientations. We used the Kalman filter to reach higher accuracy in a lower number of images. The resulting maps indicated that diffusion maps and pseudodiffusion maps for a healthy case are of highly visual similarity. Our results also confirmed that achieving a good accuracy is possible with just half number of images.
INTRODUCTION:
Intravoxel incoherent motion (IVIM)1 and diffusion tensor imaging (DTI)2 are two diffusion-weighted imaging models when their combination might result in functional and structural information. The long acquisition time and lack of accuracy limited its application in practice. The aim of this study is to combine IVIM and DTI models to simultaneously extract functional and structural information using Kalman filter such that both the accuracy of information is maintained and the acquisition time is optimized. The IVIM-DTI model is formulated as follows: $$S=fS_0 e^{(-bg ̂^T D^* g ̂ )}+(1-f)S_0 e^{(-bg ̂^T Dg ̂ )} $$ where b is the b-value; S is the vector of signal intensities at b-values and gradient vector (g) ,and S0 is the signal intensity at b=0. Diffusion and pseudodiffusion coefficient tensors are D and D*; f and (1-f) are their corresponding blood fractions within each voxel.METHODS:
Diffusion-weighted images were acquired at 11 b-values (0, 50, 150, 250, 350, 450, 550, 650, 750, 850, 1150 s/mm2 with 12 b0) and 64 orientations. The IVIM-DTI model was redefined in a state-space representation, and the Kalman filter was applied to estimate perfusion and diffusion maps for improving the accuracy of the resulting maps. We also compared the resulting maps of the hybrid IVIM-DTI model with a lower number of orientations to those of 64 orientations in order to find the minimum number of images that can provide enough accuracy.RESULTS:
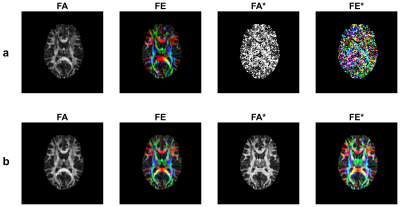
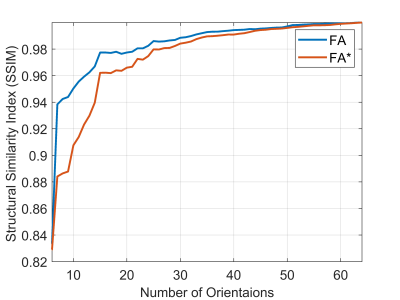
DTI and D*TI resulting maps of the proposed method and the conventional solution is depicted in Figure1, where maps of the first eigenvector (FE) of D and the first eigenvector (FE*) of D* tensors were visualized - suggesting that D*TI maps of the proposed method were significantly sharper than those derived by the conventional method. The resemblance of D*TI and DTI maps was assessed statistically by the correlation coefficient. The correlation between FA and its counterpart's FA* map was 0.97. This high rate of correlation was a confirmation of the visual similarity of these maps for a healthy case. As seen in Figure 2, when the number of orientations increased, the accuracy of FA and FA* maps improved too. The degree of similarity between DTI and D*TI maps of each slide reconstructed using the proposed method by different numbers of orientations and those of all 64 orientations as ground truth was assessed by the Structural Similarity Index (SSIM) algorithm. Results confirmed that the increase of accuracy after using 32 directions was not that significant; hence the acquisition time can be reduced by half (Figure 2).DISCUSSION:
The accuracy of maps is of great importance; the Kalman filter reaches a precise estimation by predicting the outputs using the IVIM state-space model and correcting estimates using new measurements. The resulting maps of FA and FA* are highly similar, suggesting that the distribution of nerve fibers might result in the capillary network arrangement. The long acquisition time of DTI-IVIM is critically limiting in practice. Reducing acquisition time not only reduces cost and time but also decreases patient motion artifacts. Kalman filter reduces imaging time by estimating accurately with a lower number of orientations and at the same time preserves the quality of DTI and D*TI maps as well. Of course, to achieve this goal, the accuracy of the estimate is slightly decreased. But this price for halving the acquisition time is negligible. This result is consistent with the results of Poupon et al3.CONCLUSION:
Although research about IVIM-DTI is in its early stages, it seems that computational methods such as the Kalman filter can increase its reliability and decrease the acquisition time so that a hybrid IVIM-DTI imaging would be possible in clinical practice. The hybrid IVIM-DTI imaging can reconstruct functional and structural maps that could be really useful in the study of brain diseases.Acknowledgements
No acknowledgement found.References
1. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505. doi: 10.1148/radiology.168.2.3393671. PubMed PMID: 3393671.
2.Basser PJ, Jones DK. Diffusion‐tensor MRI: theory, experimental design and data analysis–a technical review. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo. 2002;15(7‐8):456-67.
3. Poupon C, Roche A, Dubois J, Mangin JF, Poupon F. Real-time MR diffusion tensor and Q-ball imaging using Kalman filtering. Med Image Anal. 2008 Oct;12(5):527-34. Epub 2008/07/31. eng. doi: 10.1016/j.media.2008.06.004. PubMed PMID: 18664412.
Figures