1490
Assessment of Achilles Tendon Changes After Long-Distance Running Using Ultrashort Echo Time Magnetization Transfer MR Imaging1The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, China, 2MR Research, GE Healthcare, Beijing, China, 3University of California, San Diego, CA, United States
Synopsis
Long-distance running is one of the common causes of Achilles tendon injury. UTE-MT is a magic angle-insensitive MRI technique and an excellent fit for the assessment of Achilles tendon which has a highly anisotropic collagen structure.This study aims to explore the feasibility of quantitative UTE-MT imaging in the assessment of Achilles tendon changes for subjects before and after long-distance running, then compare the technique’s performance with that of UTE-T2*.
INTRODUCTION
Long-distance running is a common cause of Achilles tendinopathy. The early pathological stage of Achilles tendinopathy is considered to have some degree of reversibility given the appropriate healing environment and recovery time. A fast, reliable, and non-invasive magnetic resonance imaging (MRI) technique to track the early changes in tendon is of critical importance for effective clinical intervention and evaluation that can prevent the progression of Achilles tendinopathy. This study aims to explore the feasibility of quantitative UTE-MT imaging in the assessment of Achilles tendon changes for subjects before and after long-distance running, then compare the technique’s performance with that of UTE-T2*.METHODS
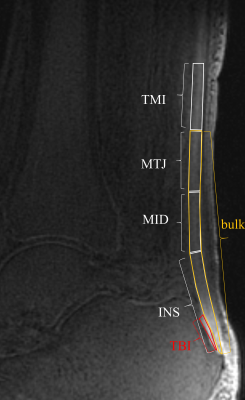
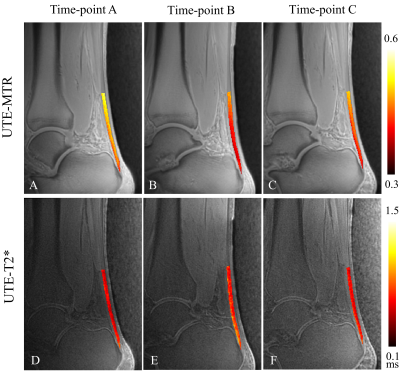
Thirty-two amateur marathon runners were recruited and had their Achilles tendons scanned with the UTE-MT sequence at three different time points: 1 week pre-, 2 days post-, and 4 weeks post-marathon race. The UTE-T2* values were also measured for comparison. The Achilles tendon was divided into six regions of interest (ROIs) for data analysis, namely the insertion part (INS), middle part (MID), muscle-tendon junction (MTJ), tendon-bone insertion (TBI), tendon-muscle insertion (TMI), and the whole Achilles tendon (bulk). One-way ANOVA and Friedman’s rank tests were used to evaluate the changes in UTE-MT ratio (UTE-MTR) and UTE-T2* between the various time points, respectively. Tukey test and the Bonferroni method were used for paired comparisons among the three different time points.RESULTS
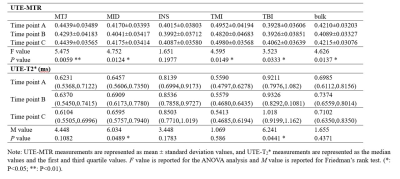
The UTE-MTR values of nearly all the tendon ROIs changed significantly between the measured time points (P<0.05), except for the INS region (P=0.1977). Conversely, the UTE-T2* values only showed significant changes in the MID and TBI regions (P<0.05). Paired comparisons showed that the UTE-MR decreases in the MTJ, MID, TMI, and bulk regions at 2 days post-race were statistically significant compared to measures taken pre-race and 4 weeks post-race (P<0.05). For UTE-T2* measurements, significant differences were observed only for the MID region between pre-race and 2 days post-race (P=0.0408), and for the TBI region between pre-race and 4 weeks post-race (P=0.0473).DISCUSSION
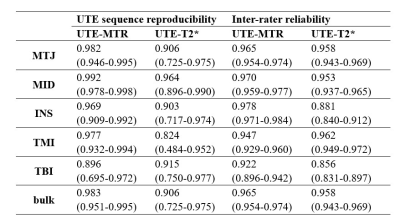
In this study, The results demonstrate that UTE-MTR may be a more useful biomarker in the detection of dynamic changes in the Achilles tendon before and after long-distance running compared to UTE-T2*. Compared to UTE-T2*, UTE-MT imaging has been found to be generally more reproducible and to generate more stable data analysis. This is probably because the UTE-MT technique is much less sensitive to the magic angle effect than UTE-T2* . It is therefore possible that a discrepancy in sample alignments (e.g., tendon fiber orientation relative to the direction of the B0 field) between different time point scans or any slight differences in ROI selection between the two raters would have produced larger changes in the UTE-T2* measurements than the UTE-MT measurements taken in this study. During long-distance running, repeated mechanical pulling of the Achilles tendon may lead to biochemical changes in the tendon such as collagen structure destruction (largely, type I collagen), proteoglycan accumulation, and the accretion of water content. This could explain the decrease of UTE-MTR values and the increase of UTE-T2* values . In the 4 weeks following a marathon, it is possible that type III collagen is produced in the tendon as a rapid “patch” that can protect the area from subsequent damage . This increase in collagen content may lead to an upward trend in the UTE-MTR values, while the associated decrease in water content may lead to the downward turn in UTE-T2* values. After 4 weeks, the UTE-MTR and UTE-T2* values were largely returned to pre-race levels. The middle part of the Achilles tendon (i.e., the MTJ and MID) is of special interest because this region is relatively low in blood supply. As a result, tendon diffuse swelling, edema, tenderness, or rupture are not only more likely to occur in this region, but injuries are relatively slower to repair than other areas in the tendon. The UTE-MTR imaging showed significant changes in this region both before and after running, demonstrating that the quantitative UTE-MTR technique may be a feasible biomarker for monitoring tendon recovery following injury or disease. Our study had several limitations. First, the sample size was relatively small, with only 32 amateur marathon runners participating. A future study with an increased sample size would improve the credibility of the statistical tests. Second, we performed single component analysis for the UTE-T2* measurements. Many previous studies have shown the advantages of bi-component analysis in the Achilles tendon. In a future study, UTE data with additional echo times should be acquired to facilitate bi-component analysis. Third, all the subjects recruited in this study were healthy runners. It would be interesting to perform a study investigating whether the UTE-MT technique is useful in evaluating biochemical changes involved in tendon disease.CONCLUSION
The UTE-MTR sequence is able to detect biochemical changes in the Achilles tendon before and after long-distance running.Acknowledgements
the National Natural Science Foundation of China (82101995 to YJ.F)References
1.Kakouris N, Yener N, Fong DTP. A systematic review of running-related musculoskeletal injuries in runners. Journal of Sport and Health Science. 2021/04/20/ 2021;
2Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. Jun 2009;43(6):409-16.
3. Ma Y-J, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed. 2016/11/01 2016;29(11):1546-1552.
4. Syha R, Martirosian P, Ketelsen D, et al. Magnetization transfer in human Achilles tendon assessed by a 3D ultrashort echo time sequence: quantitative examinations in healthy volunteers at 3T. Fortschr Rntgenstr. 2011;183(11)
5. Jerban S, Ma Y, Namiranian B, et al. Age-related decrease in collagen proton fraction in tibial tendons estimated by magnetization transfer modeling of ultrashort echo time magnetic resonance imaging (UTE-MRI). Scientific reports. Nov 29 2019;9(1):17974.
6. Chen B, Zhao Y, Cheng X, et al. Three-dimensional ultrashort echo time cones (3D UTE-Cones) magnetic resonance imaging of entheses and tendons. Magn Reson Imaging. Jun 2018;49:4-9.
7. Diaz E, Chung CB, Bae WC, et al. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed. 2012/01/01 2012;25(1):161-168.
8. Millar NL, Silbernagel KG, Thorborg K, et al. Tendinopathy. Nat Rev Dis Primers. Jan 7 2021;7(1):1. 26. Provenzano PP, Vanderby R, Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix biology : journal of the International Society for Matrix Biology. Mar 2006;25(2):71-84.
9. Chen TM, Rozen WM, Pan W-r, Ashton MW, Richardson MD, Taylor GI. The arterial anatomy of the Achilles tendon: Anatomical study and clinical implications. Clinical Anatomy. 2009/04/01 2009;22(3):377-385.
10. Maffulli N, Longo UG, Kadakia A, Spiezia F. Achilles tendinopathy. Foot and Ankle Surgery. 2020/04/01/ 2020;26(3):240-249.
11. Chiodo CP, Wilson MG. Current concepts review: acute ruptures of the achilles tendon. Foot & ankle international. Apr 2006;27(4):305-13.
Figures