1479
Motion Compensation in Pulmonary Ultra-short Echo Time MRI: Preliminary Results in Neonatal Bronchopulmonary Dysplasia1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Division of Pulmonary Medicine, Cincinnati Children’s Medical Center, Cincinnati, OH, United States, 3Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 4Department of Radiology, Cincinnati Children’s Medical Center, Cincinnati, OH, United States, 5Department of Radiology, University of Iowa, Iowa City, IA, United States
Synopsis
Self-gating of free breathing pulmonary MR images remains challenging due to inconsistent tidal breathing and bulk motion especially in neonatal patients. We evaluate several conventional and advanced retrospective motion compensation techniques in neonatal subjects with lung disease of prematurity. Registration-based techniques show a significant improvement in signal to noise ratio and contrast to noise ratio relative to other motion compensation methods. There were no significant changes in sharpness across the cohort. We conclude that registration-based techniques could be used to improve image quality without loss of sharpness.
Introduction
Bronchopulmonary Dysplasia (BPD) is a chronic pulmonary disease in infants resulting from preterm birth. BPD subjects are known to have structural abnormalities such as bronchial wall thickening and structural heterogeneity (mosaic pattern) across the lungs. Recently, 3D radial ultrashort echo-time (UTE) MRI with self-gating of respiratory motion in infants with BPD has been shown to be a strong independent predictor of short-term clinical outcomes and disease severity1. However, due to long acquisition times and inconsistent tidal breathing in disease, motion corruption is still frequently observed. Recently, more advanced motion correction techniques have been proposed to retrospectively improve image quality. The purpose of this study was to evaluate these strategies in the highly challenging setting of infants with BPD who must be imaged during tidal breading. Our goal is to identify a current best approach for compensating for respiratory motion using 3D radial UTE MRI in this population.Methods
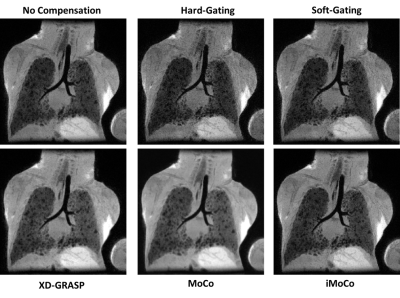
Fifteen neonatal subjects with BPD were recruited in a HIPAA-compliant and IRB approved study with parental consent and imaged with a 3D radial UTE (TE: 200 μs, TR = 4.4–5.2 ms, FA = 5° or 10°; FOV = 18 cm; 3D isotropic resolution = 0.70–0.86 mm, scan time = ∼10–16 min; and number of radial projections = ∼120,000–200,000) on a neonatal 1.5T scanner2,3. Five retrospective motion compensation techniques were considered: 1) hard thresholding of the k0 signal to 50% (hard-gating), 2) exponentially weighting of the bellows signal (soft-gating)4, 3) motion-state resolved compressed sensing reconstruction with a total variation constraint across motion states (XD-GRASP), 4) motion compensated averaging of different motion states (MoCo), and 5) an iterative motion compensation compressed sensing reconstruction technique (iMoCo).5 For XD-GRASP and iMoCo we fixed the regularization parameters to λ=0.05 and number of motion states to 8.Minimum intensity projections (MIPs) were used to qualitatively highlight relative visualization in areas of heterogeneity in BPD. Signal to noise ratio (SNR) and contrast to noise ratio (CNR) were measured within manual regions of interest in the airways, lung parenchyma, liver, muscle tissue, and aorta. CNR was computed relative to the airways as a low signal regional standard. Image sharpness was assessed by computing the maximum value of a gradient profile across the diaphragm within a region of interest. The discrete wavelet transform (DWT) was used as an automated measure of texture and sharpness across the entire image; heterogeneity of structural disease in BPD could obfuscate a localized region of interest sharpness measure, e.g., near the diaphragm. Therefore, it is desirable to have a metric that could quantify overall sharpness of the image, including the disease itself.
Results
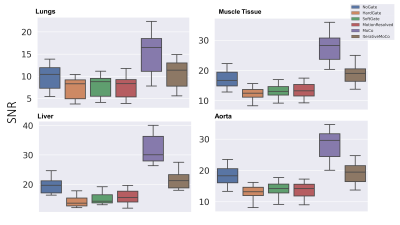
After compensating for motion using conventional gating and binning techniques (Methods 1 and 2), significant SNR is lost when compared to the corresponding non-compensated image (Fig. 1). XD-GRASP (Method 3), MoCo and iMoCo all show improved image quality with respect to contrast between the lung parenchyma and areas of alveolar simplification relative to non-compensated and gated reconstructions.Quantitative measures of SNR and CNR support the qualitative trends (Figs. 2 and 3). More specifically, conventional methods tend to have lower SNR and CNR values compared to registration-based techniques. Registration based methods MoCo and iMoCo (Methods 4 and 5) showed improved quality with respect to apparent SNR with MoCo showing significantly higher SNR and CNR than iMoCo. Sharpness, evaluated via the maximum value of the gradient of the diaphragm/lung boundary was increased in all methods relative to non-compensated reconstructions, although statistical significance was not reached. (Fig.4) Similarly, motion compensation methods trend towards higher sharpness using the wavelet-based sharpness measure, although statistical significance was not reached in any case (Fig 5). Although sharpness was not statistically different for any of the motion compensation methods tested, iMoCo trended towards higher sharpness values while showing statistically higher CNR in all tissues (p < 0.05) compared to hard gating, soft gating, and XD-GRASP.
Discussion
Preliminary results suggest a moderate improvement in image quality using motion compensation methods based on compressed sensing and registration. SNR and CNR are improved, especially for MoCo, likely due to the efficient use of all acquired data. SNR and CNR are likely higher in MoCo relative to iMoCo likely because iMoCo enforces data consistency with k-space, mitigating the artificial smoothing caused by registration and averaging. However, sharpness evaluated via the maximum gradient value at the diaphragm/lung interface and the DWT autofocus measure indicate comparable sharpness for all methods. The lack of significant differences could be due to our relatively small sample size for this ongoing study.Conclusion
Initial assessment of these motion compensation strategies suggest that significant improvement can be made using registration-based techniques such as MoCo and iMoCo with respect to SNR and CNR over conventional gating techniques. While promising, further work needs to be done to evaluate these techniques for image sharpness and robustness to different kinds of motion encountered in neonatal lung disease.Acknowledgements
The authors thank our collaborators and colleagues. This work was supported by NIH/NHLBI grants R01 HL126771, R01 HL136965, R01 HL146689, UL1TR000427 to University of Wisconsin Institute for Clinical and Translational Research (ICTR), and the University of Wisconsin Pulmonary Imaging Center (NIH S10 OD016394). This project was also supported in part through a fellowship to Luis Torres from the University of Wisconsin Science and Medicine Graduate Research Scholars Program (SciMed GRS), Parker B. Francis Foundation, Little Giraffe Foundation, and Cincinnati Children’s Research Foundation.
References
1. Higano, N. S. et al. Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes. Am. J. Respir. Crit. Care Med. 198, 1302–1311 (2018).
2. Tkach, J. A. et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr. Radiol. 42, 1347–1356 (2012).
3. Hahn, A. D. et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system: Pulmonary MRI of Neonates in the ICU. J. Magn. Reson. Imaging 45, 463–471 (2017).
4. Johnson, K. M., Block, W. F., Reeder, S. B. & Samsonov, A. Improved least squares MR image reconstruction using estimates of k-space data consistency. Magn. Reson. Med. 67, 1600–1608 (2012).
5. Zhu, X., Chan, M., Lustig, M., Johnson, K. M. & Larson, P. E. Z. Iterative motion-compensation reconstruction ultra-short TE (iMoCo UTE) for high-resolution free-breathing pulmonary MRI. Magn. Reson. Med. 0,.Figures