1474
Multi-parametric 18-FDG PET/MRI in predicting lung cancer lymphatic metastasis
Fangfang Fu1, Meng Nan2, Yaping Wu1, Zhun Huang3, Yan Bai1, Pengyang Feng3, Ting Fang2, Jianmin Yuan4, Yang Yang5, Haiyan Gao1, and Meiyun Wang1
1Henan Provincial People's Hospital & People's Hospital of Zhengzhou University, Zhengzhou, China, 2Zhengzhou University People's Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 3Henan University People's Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 4Central Research Institute, Beijing, China, 5Beijing United Imaging Research Institute of Intelligent Imaging, Beijing, China
1Henan Provincial People's Hospital & People's Hospital of Zhengzhou University, Zhengzhou, China, 2Zhengzhou University People's Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 3Henan University People's Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 4Central Research Institute, Beijing, China, 5Beijing United Imaging Research Institute of Intelligent Imaging, Beijing, China
Synopsis
It is meaningful to predict lymph node status of lung cancer before surgery. In the study, 18F-FDG PET/MRI were used to obtain PET metabolic parameters, multi-model DWI and APTWI parameters for predicting LNM of lung cancer. Our results showed that multiparametric PET/MRI is helpful in predicting lymph node status before surgery. 18F-FDG hybrid PET/MRI enables a robust diagnosis ability of LNM of lung cancer when several multi-model DWI parameters, PET metabolic parameters and APT parameter are combined. Thus, integrated PET/MRI can help to characterize LNM before surgery.
Introduction
Lymph node status is one of the most important prognostic factors for lung cancer [1,2,3]. The accurate evaluation of lymph node metastasis (LNM) is important for clinical stage and decisions and for selecting the optimal treatment for lung cancer [3,4]. Therefore, it is of great clinical value to search for early biomarkers that can be used to accurately evaluate LNM of lung cancer before surgery. With the rapid development of imaging technology, hybrid PET/MRI has been used in clinic as the most advanced equipment, and has shown great application prospect in oncology as a new imaging method [5,6,7]. However, the value of PET/MRI in predicting LNM of lung cancer remains to be further explored. Thus, the aim of the study is to evaluate the clinical utility of multiparametric 18F-FDG PET/MRI in predicting lymph node status before surgery and compare the diagnostic efficiency of various quantitative parameters obtained from PET, diffusion weighted imaging (DWI) and amide proton transfer-weighted imaging (APTWI) in predicting LNM of lung cancer.Methods
Between July 2020 and October 2021, a total of 79 patients with lung cancer were confirmed by postoperative pathological examination with non LNM and LNM (LNM group (40) and no LNM group (39)). All the patient underwent preoperative PET/MRI by using a hybrid 3.0T PET/MR scanner (uPMR 790; UIH, Shanghai, China) with a 12 channels phased-array body coil in the supine position. The PET/MRI images were acquired 40-60 minutes of quiet rest after intravenous injection according to the standard dose of 0.15 mCi per kilogram weight. DWI with multiple b-values was performed using a single-shot spin-echo planar sequence and was set with the following parameters: TR= 1620 ms, TE= 69.6 ms. slice thickness, 5 mm; intersection gap, 1 mm. Thirteen different b-values were used: 0, 25, 50, 100, 150, 200, 400, 600, 800 and 1000 sec/mm2. For APTWI: ETL = 39, B1 = 1.3 μT and 2.5 μT, Gaussian pulse, 100 ms duration, 10 repeats, Δ spanned from [-4.5 4.5] ppm in 31 steps, plus one S0 with no CEST saturation pulse for normalization; 11 low power B1 = 0.13 μT, Δ spanned from [-1.0 1.0] ppm images were collected as WASSAR images for B0 map correction. Metabolic parameters including SUVmax, TLG and MTV were obtained from PET; The standard apparent diffusion cofficient (sADC), pseudo-diffusion coefficient (D*), true diffusion coefficient (D), perfusion fraction (F), distributed diffusion coefficient (DDC) and water molecular diffusion heterogeneity index (α) were calculated from multi-model DWI; the magnetization transfer ratio asymmetry (MTRasym (3.5 ppm)) were calculated from APT. All statistical analyses were performed using SPSS 25.0 and MedCalc12.0. The Kolmogorov-Smirnov test was used to determine whether the quantitative data conformed to a normal distribution. The t test or the Mann-Whitney U test, were used to determine the differences in each parameter between the LNM group and no LNM group. ROC curves were used to assess the diagnostic efficacy of each parameter. P values less than 0.05 were considered significantly different.Results
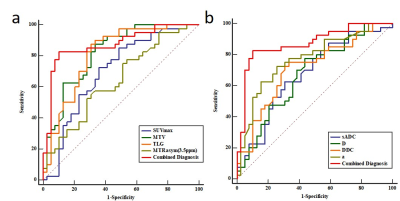
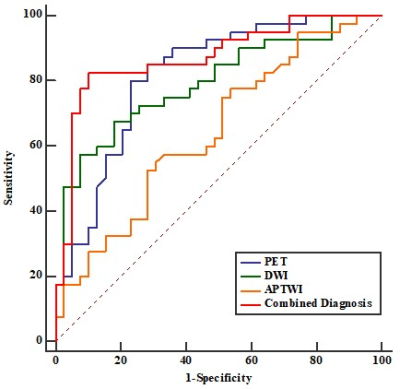
D, DDC and sADC values were lower (all P<0.05), while SUVmax, TLG, MTV, α, MTRasym (3.5 ppm) were higher in LNM group (all P<0.05) compared with no LNM group. Both D* and F showed no significant difference between LNM group and no LNM group (P>0.05). In predicting LNM, the combined diagnosis (SUVmax+ TLG+ MTV+ sADC+ D+ α+ DDC+ MTRasym) showed the highest AUC (0.871), followed by TLG (0.821), MTV (0.810), α (0.769), DDC (0.712), SUVmax (0.699), sADC (0.675), D (0.674), and the differences between the combined diagnosis and other parameters were significant (all P<0.05) except for TLG and MTV (Figure1). Additionally, in predicting LNM of lung cancer, the PET ((SUVmax+TLG+MTV) showed the highest AUC (0.814), followed by DWI (sADC+D+α+DDC) (0.794), APTWI(MTRasym) (0.642). (Figure2). There was significant difference between PET and APTWI for predicting LNM (P<0.05), whilst there was no significant difference between PET and DWI, between DWI and APTWI (both P >0.05).Discussion
The results of the study demonstrated that D, DDC and sADC values were lower, whilst SUVmax, TLG, MTV, α, MTRasym (3.5 ppm) were higher in LNM group than that in no LNM group. However, both Dp and F showed no statistically significant difference between LNM group and no LNM group, implying that they might not be a potentially useful parameter for predicting LNM. Additionally, PET, DWI and APTWI parameters could be used as independently quantitative indexes and have potential value for predicting LNM of lung cancer. Moreover, the combination of PET, DWI and APTWI showed highest diagnostic performance for predicting LNM. Thus, hybrid PET-MRI can be used as a potential imaging method to forecast lymph node status of lung cancer.Conclusions
The PET, DWI and APTWI parameters can be used as reliable imaging markers to forecast LSM of lung cancer before surgery. Moreover, the combination of the above parameters could improve the performance for predicting LSM.Acknowledgements
This research was supported by Zhengzhou Collaborative Innovation Major Project (20XTZX05015), Key Project of Henan Province Medical Science and Technology Project (LHGJ20210001) (LHGJ20190602)(LHGJ20210005) ,Henan provincial science and technology research projects (212102310689)References
1.Imai K, Minamiya Y, Saito H, et al. Diagnostic imaging in the preoperative management of lung cancer[J]. Surgery Today, 2014, 44(7): 1197-1206. 2. Verhagen AF, Bulten J, Shirango H, et al. The clinical value of lymphatic Micrometastases in patients with non-small cell lung cancer. J Thorac Oncol, 2010, 5(8): 1201-120 3.Dziedzic D, Peryt A, Szolkowska M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the staging of lung cancer patients[J]. SAGE Open Med, 2015, 3(3): 1-6. 4. Chansky K, Detterbeck F C, Nicholson A G, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer[J]. J Thorac Oncol, 2017, 12(7): 1109-1121 5.Schulz V, Torres-Espallardo I, Renisch S, et al. Automatic, three-segment, MR-based attenuation correction for wholebody PET/MR data. Eur J Nucl Med Mol Imaging. 2011; 38(1): 138-152. 6.Sawicki LM, Grueneisen J, Buchbender C, et al. Comparative performance of 18F-FDG PET-MRI and 18FFDG PET-CT in detection and characterization of pulmonary lesions in 121 oncologic patients. J Nucl Med. 2016; 57(4): 582-586 7.Chandarana H, Heacock L, Rakheja R, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology.2013;268(3): 874-881.
DOI: https://doi.org/10.58530/2022/1474