1465
Automatic spinal cord segmentation in pediatric MR images1NeuroPoly Lab, Institute of Biomedical Engineering, Polytechnique Montréal, Montreal, QC, Canada, Montreal, QC, Canada, 2Research Center, Ste-Justine Hospital University Centre, Montreal, QC, Canada, Montreal, QC, Canada, 3Thomas Jefferson University, Philadelphia, PA, United States, Philadelphia, PA, United States, 4Department of Computer and Software Engineering, Polytechnique Montréal, Montréal, QC, Canada, Montreal, QC, Canada
Synopsis
Segmentation of the spinal cord is an essential process for the accurate delineation of spinal cord structures. However, it is a long process and automatic segmentation tools are not adapted to segment the pediatric spinal cord. We therefore developed a tool mixing a neural network and a deterministic method to overcome the limitations. We succeeded in obtaining a segmentation with a dice coefficient of 0.86 on a patient with a spinal cord injury (SCI).
Introduction
During a child's normal development, the various neural and structural tissues form at varying rates. The spinal cord, essential to the proper functioning of the central nervous system, grows in a non-linear way in space1. Recent studies have demonstrated the potential of spinal cord imaging and the significance of quantitation imaging to study progression of degenerative diseases affecting the central nervous system, as a result of traumatic or non-traumatic spinal cord injury (SCI) 2,3. However, Magnetic resonance imaging (MRI) tools of the spinal cord have been developed with healthy adults in mind, and are therefore not necessarily optimized to the anatomy of pediatric populations 4 or to specific pathologies such as syringomyelia. In these cases, a proper segmentation of the spinal cord must be obtained manually, which is subjective and time consuming.Providing clinicians with an automated segmentation tool tailored to a new population would save time but also increase accuracy and robustness. We observe that from one expert to another the segmentation is done in a different way6. Using the same tool for the segmentation during the treatment of a patient, no matter the age, would allow a better homogeneity of the segmentations and thus the extraction of more robust quantitative data for disease monitoring. The development of an automatic spinal cord segmentation tool adapted to pediatric populations would therefore have several advantages: time saving for experts, increased accuracy and therefore ease of follow-up for the treatment of pathologies.In this study, we developed an automatic spinal cord segmentation method adapted to a pediatric population or with pathologies significantly affecting the spinal cord structure, such as degenerative cervical myelopathy.
Methods
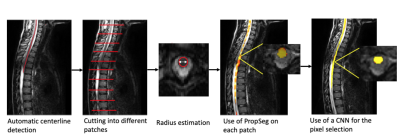
High-resolution T2-weighted MR images were acquired from 15 typically-developing children and 1 children with spinal SCI, covering the cervical and thoracic, on a 3T MRI scanner. The proposed method uses a local segmentation approach that combines a deterministic method (PropSeg) on overlapping small patches and a supervised machine learning approach for merging all segmentation results from patches to produce a global segmentation (Figure 1).As a first step, we chose to repurpose the PropSeg spinal cord segmentation algorithm5. This method works by propagating a deformable model along the spinal cord and was developed from a population of healthy adults. In order to overcome the error propagation problem inherent to global surface propagation, we employed a local approach by partitioning the spinal cord into multiple overlapping patches with variable initialization parameters.
In a second step, the results from the segmentation of each overlapping patch were aggregated into a single segmentation. Then, we have to refine our segmentation by selecting the pixels. For this selection we have developed a method based on a fully convolutional network with a U-Net architecture that allows us to create a complete and consistent segmentation of the spinal cord. As input we chose the layering of the MR images and the first segmentations of 13 healthy patients. The network gives us the final segmentation for our images.
We chose to validate our model with 2 healthy subjects and 1 subject with spinal cord injury (SCI). We calculated the dice coefficient based on a manual segmentation. We also compared our method with the PropSeg algorithm.
Results
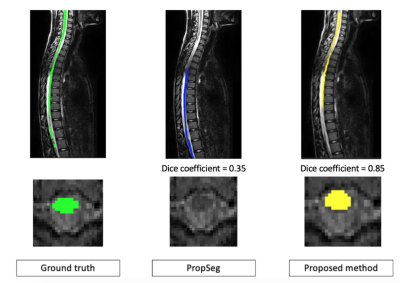
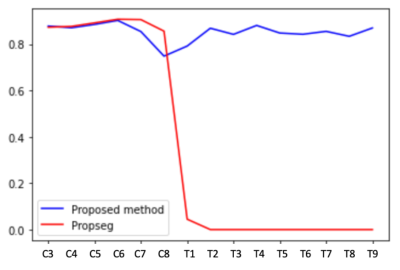
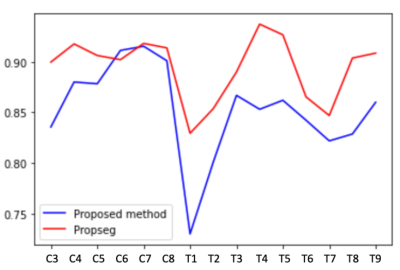
With the proposed method, our results on two healthy patients show a dice coefficient of 0.86 versus 0.89 with PropSeg. In a single SCI patient, the mean dice coefficient was 0.85 versus 0.35 with PropSeg (Figures 2 and 3). Figure 4 shows the Dice scores along the spinal cord for all subjects for the proposed method and PropSeg.Discussion / conclusion
In this preliminary study, we demonstrated the robustness of a local approach for segmenting the spinal cord on pediatric MR images and on images with low contrasts and injuries. Overall results show that the proposed method provides good segmentation results, although slightly less accurate than the original PropSeg method on healthy subjects.However, we demonstrated the potential of the local segmentation approach on patients with SCI. Efficient automatic segmentation allows precise extraction of quantitative data from patients. The exploitation of this data could allow in the future to assist clinicians in the follow-up of pathologies in patients or even in the programming of operations. Future studies with a large number of patients is warranted. The combination of a deterministic algorithm and artificial intelligence allows the operator to modify many initialization parameters and so to adapt to a large number of patients while surpassing the limitations of PropSeg.
Acknowledgements
This study was supported by Polytechnique Montréal, by the Canada First Research Excellence Fund, and by the TransMedTech Institute.References
1. L. C. Vogel, R. R. Betz, and M. J. Mulcahey, “Chapter 8 - Spinal cord injuries in children and adolescents,” in Handbook of Clinical Neurology, vol. 109, J. Verhaagen and J. W. McDonald, Eds. Elsevier, 2012, pp. 131–148.
2. Y. Cohen, D. Anaby, and D. Morozov, “Diffusion MRI of the spinal cord: from structural studies to pathology,” NMR Biomed., vol. 30, no. 3, Mar. 2017.
3. M. I. Vargas, J. Boto, and T. R. Meling, “Imaging of the spine and spinal cord: An overview of magnetic resonance imaging (MRI) techniques,” Rev. Neurol. , Aug. 2020.
4. B. De Leener, S. Lévy, S. M. Dupont, V. S. Fonov, N. Stikov, D. Louis Collins, V. Callot, and J. Cohen-Adad, “SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data,” Neuroimage, vol. 145, no. Pt A, pp. 24–43, Jan. 2017.
5. B. De Leener, S. Kadoury, and J. Cohen-Adad, “Robust, accurate and fast automatic segmentation of the spinal cord,” Neuroimage, vol. 98, pp. 528–536, Sep. 2014.
6. Prados F, Ashburner J, Blaiotta C, Brosch T, Carballido-Gamio J, Cardoso MJ, et al. Spinal cord grey matter segmentation challenge. Neuroimage, vol. 152, pp. 312–329, May 2017.
Figures