1464
Early aging, iron accumulation and demyelination in cervical spinal cord and brain as long-term effects of rugby practice?1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hopital Universitaire Timone, CEMEREM, Marseille, France, 3Aix-Marseille Univ, Université Gustave Eiffel, LBA, Marseille, France, 4iLab-Spine International Associated Laboratory, Marseille-Montreal, Marseille, France, 5APHM, Hopital Universitaire Nord, Neurosurgery Dept, Marseille, France, 6Siemens Healthcare SAS, Saint-Denis, France, 7APHM, Hopital Universitaire Timone, Pole MPR, Marseille, France
Synopsis
Brain alterations due to cumulative effects of impacts have been reported in rugby players. However, literature on long-term effects is scarce, and no study has been conducted to characterize potential spinal cord impairments.
In this study using a multiparametric MR protocol dedicated to both brain and cord, and combining state-of-the-art T1 relaxometry and ihMT imaging, we observed diffuse T1 increase in the cervical spinal cord, together with increased T1 and decreased ihMTsat in specific brain WM tracts in retired players.
These preliminary results also suggest early aging, tissue degeneration, iron accumulation, and different aging processes in retired players.
Introduction
Players of contact sports such as rugby receive multiple impacts to their head and neck1 over their career.Adverse effects have been demonstrated in such players as compared to non-contact players such as altered functional connectivity2 and changes of brain microstructure characterized by decreased Fractional Anisotropy in multiple white matter (WM) tracts3, along with more chronic neck pain and narrower vertebral canal on spine4,5. However, no studies have been conducted so far to characterize potential effects on the cervical spinal cord (cSC).
In this study, we propose to use complementary state-of-the-art quantitative MRI techniques that have been recently adapted to brain and cSC imaging at 3T, to scan retired rugby players, investigate whether the cord is impaired and to refine brain structure damage description. The protocol included fast T1 mapping, which has been used widely to study tissue alterations such as demyelination, iron deposition or structural variations in neurodegenerative diseases6–8, and inhomogeneous Magnetization Transfer (ihMT)9, a technique specific to the myelinated tissue10, which has been used in several studies on brain11–13 and SC to study demyelinating pathologies such as multiple sclerosis14,15 or normal aging16,17.
Materials & Methods
Data were acquired from 15 retired amateur and professional rugby players (R) and 15 age-matched healthy controls (HC) (all males; mean age R: 46.8±7.6 years, mean practice duration=26.2±9.5 years, mean duration from retirement=9.8±5.4 years; mean age HC: 48.6±9.5 years) using a 3T MR system (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) with a 20-channel head and neck coil.The MRI protocol included conventional sequences (FLAIR, T2 TSE, MGE), as well as: a 0.9-mm isotropic resolution simultaneous brain and cSC T1 Magnetization Prepared 2 Rapid Acquisition Gradient Echo (MP2RAGE) sequence18 as recently proposed in 19; a prototype 3D ihMT-RAGE sequence with a 2-mm isotropic resolution for brain and a 0.9×0.9×10-mm3 resolution for cSC as in Ref. 20–22; and a B1+ mapping based on a pre-saturated turbo flash sequence21 for correcting quantitative T1 and ihMT maps.
Post-processing & Statistical analysis
The main post-processing steps are depicted in Figure 1. Briefly, T1 data were corrected for B1+ inhomogeneity using the method introduced by Massire et al.23. Since the ihMTR and MTR metrics (obtained by the ihMT technique) can be influenced by T1 relaxation times and B1+ inhomogeneity22,24,25, a correction method that compensates for these biases hereafter referred to as ihMTsat and recently adapted to the ihMT-RAGE framework25,26, was also adopted and computed according to:$$\text{ihMT}_\text{sat} = (\delta_{MT^\pm} + \delta_{MT^\mp} - \delta_{MT^+} - \delta_{MT^-}) \times \left(\frac{B_{1,act}}{B_{1,nom}}\right)^2$$
where factor δ is related to the attenuation of the water pool for the single- (+,-) and the dual- frequency offset acquisitions ($$$\pm$$$,$$$\mp$$$, and B1nom and B1act represent the nominal and actual pulse amplitudes (post-processing pipeline: https://github.com/lsoustelle/ihmt_proc (hash f3f49e0)).
Quantitative T1 and ihMTsat maps were registered to the ICBM-MNI-15227,28 template for the brain and to the PAM50 template29 for the SC. PAM50 masks and JHU ICBM-DTI-8130 WM masks were used for quantification on SC and brain, respectively. Normalized brain GM and WM volumes and cord cross-sectional areas of the SC were also measured using SPM12 “New segment” tool31 and the SCT32.
Statistical analyses were done using JMP9 (SAS Institute Inc., Cary, NC). For comparing the quantitative measurements between the rugby players (R) and HC, the non-parametric Steel-Dwass all pairs test was used and a p-value of less than 0.05 was considered as statistically significant.
Results & Discussion
Representative images and quantitative maps obtained from one rugby player are provided on Figure 2.On SC (Fig.3a), T1 values in GM-ant-int, WM CST, PST, and RST, were significantly higher for rugby players as compared to HC, however, no differences were observed for ihMTsat, suggesting tissue modification but not necessarily demyelination. On specific WM regions of brain (Fig.3b), T1 values were significantly higher and ihMTsat significantly lower for rugby players as compared to HC, suggesting demyelination effects. No significant differences on mean metrics were observed on brain GM.
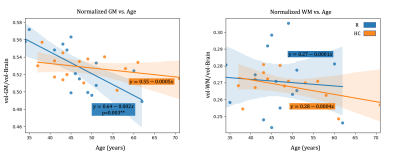
Different processes were observed in rugby players as compared to HC when comparing evolution of the metrics with age. In rugby players, T1=f(Age) had a negative slope in brain and SC GM (Fig.4) and a positive slope in HC. These different trends with age could be suggestive of iron accumulation, potentially as a result of microhemorrhages33.
Interestingly, in brain WM of rugby players an early drop of ihMTsat (corresponding to ihMTsat values observed in the oldest HC) which becomes stable over time, was concomitant with a negative slope of T1=f(Age), suggesting early demyelination followed by restructuration of the tissue involving microglia34.
Age also had a significant effect on GM volume in rugby players (Fig.5; not observed on HC, nor in WM), although on average, no significant volume differences were noticed between the two groups. The CSA of the cord was lower on all levels for rugby players, but without statistical significance.
Conclusion
In this study, early aging and structural degeneration of cSC, together with demyelination, iron accumulation and different aging processes on brain has been shown in retired rugby players as compared to age-matched controls, which could be due to cumulative effect of impacts. These preliminary observations, which may increase the likelihood of neurodegenerative diseases33,35–39, should be further investigated on larger cohorts and multicentric longitudinal studies.Acknowledgements
This work was supported by the Institut Carnot Star, with the financial support of A*MIDEX (n° ANR-11-IDEX-0001-02), funded by the Investissements d'Avenir project by the French government.References
1. Bathgate A, Best JP, Craig G, Jamieson M. A prospective study of injuries to elite Australian rugby union players. Br J Sports Med. 2002;36(4):265-269. doi:10.1136/bjsm.36.4.265 SMASH
2. Guell X, Arnold Anteraper S, Gardner AJ, et al. Functional Connectivity Changes in Retired Rugby League Players: A Data-Driven Functional Magnetic Resonance Imaging Study. J Neurotrauma. 2020;37(16):1788-1796. doi:10.1089/neu.2019.6782 SMASH
3. Manning KY, Brooks JS, Dickey JP, et al. Longitudinal changes of brain microstructure and function in nonconcussed female rugby players. Neurology. 2020;95(4):E402-E412. doi:10.1212/WNL.0000000000009821 SMASH
4. Berge J, Marque B, Vital JM, Sénégas J, Caillé JM. Age-related changes in the cervical spines of front-line rugby players. Am J Sports Med. 1999;27(4):422-429. doi:10.1177/03635465990270040401 SMASH
5. Brauge D, Delpierre C, Adam P, Sol JC, Bernard P, Roux FE. Clinical and radiological cervical spine evaluation in retired professional rugby players. J Neurosurg Spine. 2015;23(5):551-557. doi:10.3171/2015.1.SPINE14594 SMASH
6. Haacke EM, Cheng NYC, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23(1):1-25. doi:10.1016/j.mri.2004.10.001 SMASH
7. Sian-Hülsmann J, Mandel S, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem. 2011;118(6):939-957. doi:10.1111/j.1471-4159.2010.07132.x SMASH
8. Paul F. Pathology and MRI: exploring cognitive impairment in MS. Acta Neurol Scand. 2016;134(July):24-33. doi:10.1111/ane.12649 SMASH
9. Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614-622. doi:10.1002/mrm.25174 SMASH
10. Duhamel G, Prevost VH, Cayre M, et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage. 2019;199(May):289-303. doi:10.1016/j.neuroimage.2019.05.061 SMASH
11. Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614-622. doi:10.1002/mrm.25174 SMASH
12. Girard OM, Prevost VH, Varma G, Cozzone PJ, Alsop DC, Duhamel G. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med. 2015;73(6):2111-2121. doi:10.1002/mrm.25330 SMASH
13. Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med. 2018;79(5):2607-2619. doi:10.1002/mrm.26907 SMASH
14. E. Van Obberghen et al., “Inhomogeneous Magnetization Transfer (ihMT) in normal-appearing tissue correlates with disability of multiple sclerosis patients. (P4.360),” Neurology, vol. 88, no. 16 Supplement, p. P4.360, 2017.
15. Rasoanandrianina H, Demortière S, Trabelsi A, et al. Sensitivity of the Inhomogeneous Magnetization Transfer Imaging Technique to Spinal Cord Damage in Multiple Sclerosis. AJNR Am J Neuroradiol. 2020;41(5):929-937. doi:10.3174/ajnr.A6554 SMASH
16. Taso M, Girard OM, Duhamel G, et al. Tract-specific and age-related variations of the spinal cord microstructure: A multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 2016;29(6):817-832. doi:10.1002/nbm.3530 SMASH
17. Rasoanandrianina H, Duhamel G, Feiweier T, et al. Regional and structural integrity of the whole cervical spinal cord using 3D-T1 MP2RAGE and multi-slice multi angle DTI and ihMT sequences at 3T : preliminary investigations on age-related changes . In: Proc. Intl. Soc. Mag. Reson. Med. 25. Vol 36. ; 2017:7-10.
18. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002 SMASH
19. Forodighasemabadi A, Rasoanandrianina H, El Mendili MM, Guye M, Callot V. An optimized MP2RAGE sequence for studying both brain and cervical spinal cord in a single acquisition at 3T. Magn Reson Imaging. 2021;84(September):18-26. doi:10.1016/j.mri.2021.08.011 SMASH
20. Varma G, Munsch F, Burns B, et al. Three‐dimensional inhomogeneous magnetization transfer with rapid gradient‐echo (3D ihMTRAGE) imaging. Magn Reson Med. 2020;(April):mrm.28324. doi:10.1002/mrm.28324 SMASH
21. Troalen T, Callot V, Varma G, Guye M, Alsop DC, Girard OM. Cervical Spine inhomogeneous Magnetization Transfer ( ihMT ) Imaging Using ECG-Triggered 3D Rapid Acquisition Gradient-Echo ( ihMT-RAGE ). ISMRM. 2018:3-7.
22. Forodighasemabadi A, Troalen T, Soustelle L, Duhamel G, Girard O, Callot V. Towards minimal T1 and B1 contributions in cervical spinal cord inhomogeneous magnetization transfer imaging. In: Proc. Intl. Soc. Mag. Reson. Med. ; 2020:1175.
23. Massire A, Taso M, Besson P, Guye M, Ranjeva JP, Callot V. High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. Neuroimage. 2016;143:58-69. doi:10.1016/j.neuroimage.2016.08.055 SMASH
24. Varma G, Munsch F, Girard OM, Duhamel G, Alsop DC. An inhomogeneous magnetization transfer ( ihMT ) quantification method robust to B1 and T1 variations in magnetization prepared acquisitions. ISMRM. 2019:2-5. doi:10.1016/j.jmr.2018.08.004 SMASH
25. Munsch F, Varma G, Taso M, et al. Characterization of the cortical myeloarchitecture with inhomogeneous Magnetization Transfer imaging (ihMT). Neuroimage. 2020;225(October 2020):117442. doi:10.1016/j.neuroimage.2020.117442 SMASH
26. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60(6):1396-1407. doi:10.1002/mrm.21732 SMASH
27. Fonov V, Evans A, McKinstry R, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. doi:10.1016/s1053-8119(09)70884-5 SMASH
28. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313-327. doi:10.1016/j.neuroimage.2010.07.033 SMASH
29. De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165(July 2017):170-179. doi:10.1016/j.neuroimage.2017.10.041 SMASH
30. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-582. doi:10.1016/j.neuroimage.2007.12.035 SMASH
31. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839-851. doi:10.1016/j.neuroimage.2005.02.018 SMASH
32. De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145(October 2016):24-43. doi:10.1016/j.neuroimage.2016.10.009 SMASH
33. Daglas M, Adlard PA. The Involvement of Iron in Traumatic Brain Injury and Neurodegenerative Disease. Front Neurosci. 2018;12(December). doi:10.3389/fnins.2018.00981 SMASH
34. Loane DJ, Byrnes KR. Role of Microglia in Neurotrauma. Neurotherapeutics. 2010;7(4):366-377. doi:10.1016/j.nurt.2010.07.002 SMASH
35. Zhang J, Zhang Y, Wang J, et al. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: An in vivo MR study. Brain Res. 2010;1330:124-130. doi:10.1016/j.brainres.2010.03.036 SMASH
36. Barbosa JHO, Santos AC, Tumas V, et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2*. Magn Reson Imaging. 2015;33(5):559-565. doi:10.1016/j.mri.2015.02.021 SMASH
37. Bergsland N, Tavazzi E, Laganà MM, et al. White Matter Tract Injury is Associated with Deep Gray Matter Iron Deposition in Multiple Sclerosis. J Neuroimaging. 2017;27(1):107-113. doi:10.1111/jon.12364 SMASH
38. Oshiro S, Morioka MS, Kikuchi M. Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv Pharmacol Sci. 2011;2011. doi:10.1155/2011/378278 SMASH
39. Kwan JY, Jeong SY, van Gelderen P, et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: Correlating 7 tesla MRI and pathology. PLoS One. 2012;7(4). doi:10.1371/journal.pone.0035241 SMASH
Figures
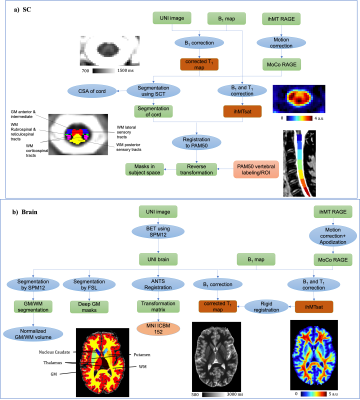
Figure 1: Main post-processing steps (B1+ and/or T1 bias-correction, segmentation, ROI labeling) for MP2RAGE and ihMT images on both a) SC and b) brain.
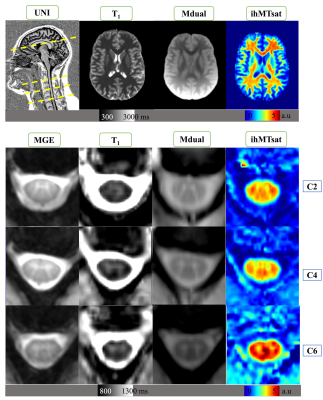
Figure 2: Representative SC and brain images acquired on one rugby player (top: sagittal UNI MP2RAGE showing both brain and cervical cord; quantitative T1 map, MT dual-offset saturation and ihMTsat acquired mid-brain; bottom: T2* MGE, T1 map, MT dual and ihMTsat acquired at C2, C4 and C6 levels).
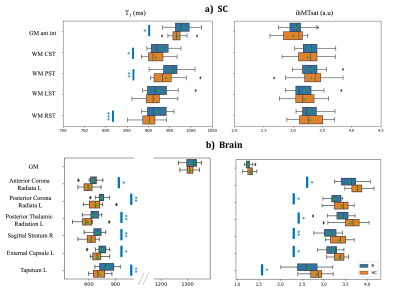
Figure 3: Boxplots of T1 and ihMTsat values in different ROIs of SC and Brain (cf. Fig.1 for ROI location) for rugby players (R in blue) and HC (orange). The green triangle shows the mean and the blue stars indicate the significance of difference between the two groups (p-value<0.05*; <0.01**; <0.001***). The brain WM tracts shown here are significantly different for both T1 and ihMTsat and have been chosen from 48 different JHU WM tracts. CST: Corticospinal Tracts, PST: Posterior Sensory Tracts, LST: Lateral Sensory Tracts, RST: Rubro & Reticulospinal Tracts; L: left, R: right.
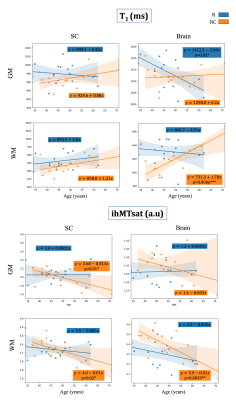
Figure 4: Linear regression plots and equations (along with MANOVA p-value if <0.05) for evolution of T1 and ihMTsat with age in GM and WM of brain and SC in R (blue) and HC (orange). The decrease of T1 in brain and SC GM of the players could demonstrate an iron accumulation effect because of impacts. The decrease of T1 and lower decrease of ihMTsat on brain WM of players with age (compared to HC) may suggest tissue restructuration (such as gliosis) following impairments.