1462
Comparison of Physiological Noise Models for Thermal Stimulus fMRI in the Cervical Spinal Cord at 7T1Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Graduate School of Biomedical Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Department of Physics, Norwegian University of Science and Technology (NTNU), Trondheim, Norway
Synopsis
A physiological noise model (PNM) is crucial for maximizing sensitivity and specificity to activation in 7T spinal cord fMRI. The PNM must balance exhaustive modeling of non-BOLD signal, preservation of degrees of freedom, and avoidance of nuisance regressors that may be correlated with the task. We compared four candidate PNMs. A 37-term PNM and a 35-term PNM excluding heart rate and respiratory volume per unit time, which may be correlated with the task, performed approximately equally well. A 32-term PNM containing only regressors derived from physiological recordings, and a 3-term PNM containing only image-derived regressors, were also evaluated.
Introduction
The spinal cord contains many neural circuits of scientific and clinical interest1, but fMRI of the spinal cord is particularly vulnerable to signal fluctuations due to respiration, motion, and pulsatile rostro-caudal flow of cerebrospinal fluid (CSF)2,3. Higher field strengths yield greater SNR and finer spatial resolution, and increases the microvascular proportion of BOLD signal in gradient-echo EPI4,5. However, higher field strengths also increase the magnitude of temporal fluctuations in B0 due to respiration6,7.A suitable physiological noise model (PNM) is crucial for maximizing sensitivity to activation in the spinal cord while rejecting non-BOLD signal contributions8,9. A balance must be struck between exhaustive modeling of non-BOLD signal, preserving degrees of freedom, and avoiding nuisance regressors which may be correlated with the task. In this work, we image activation in the cervical spinal cord produced by a noxious thermal stimulus using three 7T BOLD fMRI protocols, calculate correlations among components of a 37-term physiological noise model and the task, and compare the results of generalized linear model (GLM) analyses using four candidate physiological noise models.
Methods
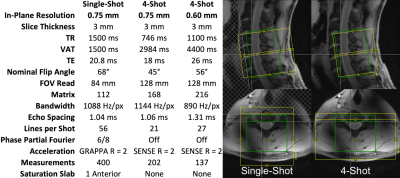
The cervical spinal cords (C4-C7 vertebral levels) of six healthy volunteers were scanned using a 7T whole-body MRI system (Magnetom, Siemens) and a 22-channel RF coil10. One single-shot EPI and two multi-shot EPI acquisition protocols were used (Figure 1). Pulse-oximeter and respiratory traces were acquired. Multi-shot reconstruction included a navigator-based per-shot frequency offset demodulation to mitigate respiratory field variations.In each 10min experiment, noxious thermal stimulation was applied to the lateral surface of the base of the right thumb at a calibrated intensity of 3/10 using an fMRI-compatible thermal stimulator (TSA-II, Medoc). This stimulus should produce sensory activation in the ipsilateral dorsal horn at the neurological C6 (vertebral C5) level. Subjects were coached to remain still and maintain steady respiration during thermal stimuli.
Images were motion-corrected slicewise (x- and y-translation) using FSL FLIRT11, straightened using Spinal Cord Toolbox12, and smoothed using an anisotropic Gaussian kernel (2mm in-plane, 6mm through-slice). GLM analysis was performed in FSL FEAT13, using four candidate PNMs: a 37-term “FullPNM” (8 cardiac phase, 8 respiratory phase, 16 cardiac-respiratory interaction9, heart rate (HR)14, respiratory volume per time (RVT)15, CSF signal, and 2 motion correction terms)8,9, a 35-term “NoRvtHr” (FullPNM minus HR and RVT), a 32-term “CardRespOnly” (8 cardiac phase, 8 respiratory phase, 16 cardiac-respiratory interaction), and a 3-term “CsfMcOnly” (CSF signal, and 2 motion correction terms). Temporal correlations among PNM components and task were calculated as the coefficient of determination (Pearson’s r2) for each acquisition, and averaged across subjects.
Results
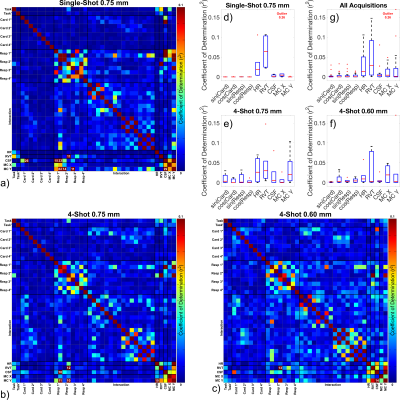
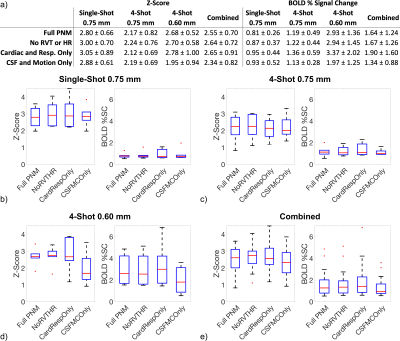
Figure 2a-c displays correlations among pairs of PNM components and the task, and Figure 2d-g plots the fraction of variance in the task that is predictable by several strongly-correlated PNM components. HR and RVT are correlated with the task with r2 approaching 5%, while CSF and motion correction terms are correlated with task with r2 only on the order of ~1%. CSF and motion correction terms are correlated with respiration, and CSF is also correlated with the cardiac cycle. Cardiac and respiratory phase terms are negligibly correlated with task.Results of GLM analyses are shown in Figure 3. Relative to FullPNM, NoRvtHr consistently yields slightly higher z-scores. CardRespOnly yields slightly higher z-scores, but z-scores and BOLD percent signal change are more variable than FullPNM or NoRvtHr. CsfMcOnly yields lower z-scores and BOLD percent signal changes.
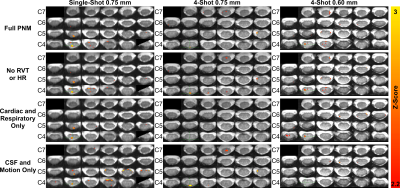
Activation maps in a single representative subject are shown in Figure 4. Relative to FullPNM, the NoRvtHr PNM introduces a small number of additional false positive voxels. CardRespOnly and CsfMcOnly yield smaller numbers of significantly activated voxels. CardRespOnly yields the fewest false positive voxels, while CsfMcOnly yields the most.
Discussion
NoRvtHr may outperform FullPNM, although the margin is small, while CardRespOnly is variable and CsfMcOnly underperforms. The superiority of FullPNM and NoRvtHr are consistent with recommendations at 3T8,9.RVT and HR were expected to correlate with the noxious thermal task. Based on these assumptions, NoRvtHr was expected to outperform FullPNM by avoiding PNM components correlated with task. Also, the CsfMcOnly PNM was included in this comparison because it is derived entirely from imaging data rather than physiological monitoring equipment, and contains cardiac and respiratory information through the cardiac cycle’s effect on CSF signal, and respiration’s effect on both CSF and motion. Generation of regressors directly from imaging data was expected to be particularly useful for multi-shot analyses, where data for a single temporal image frame is acquired in four shots at different phases of the cardiac and respiratory cycles. However, CsfMcOnly performed poorly, suggesting that an aggressively parsimonious PNM that maximizes degrees of freedom unnecessarily sacrifices terms that more fully explain non-BOLD signal contributions even in those additional cardiac and respiratory terms are not linked one-to-one with each temporal image frame.
Conclusions
In 7T cervical spinal cord task fMRI, a comprehensive PNM is valuable, although terms that may be correlated with the task should be used with caution.Acknowledgements
This study was supported by National Institutes of Health (NINDS) award number K01NS105160 (ACS).References
1. Stroman, P. W. et al. The current state-of-the-art of spinal cord imaging: methods. NeuroImage 84, 1070–1081 (2014).
2. Krüger, G. & Glover, G. H. Physiological noise in oxygenation-sensitive magnetic resonance imaging: Physiological Noise in MRI. Magn. Reson. Med. 46, 631–637 (2001).
3. Cohen-Adad, J. Functional Magnetic Resonance Imaging of the Spinal Cord: Current Status and Future Developments. Semin. Ultrasound CT MR 38, 176–186 (2017).
4. Yacoub, E. et al. Imaging brain function in humans at 7 Tesla. Magn. Reson. Med. 45, 588–594 (2001).
5. Gati, J. S., Menon, R. S., Ugurbil, K. & Rutt, B. K. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn. Reson. Med. 38, 296–302 (1997).
6. Hutton, C. et al. The impact of physiological noise correction on fMRI at 7 T. NeuroImage 57, 101–112 (2011).
7. Triantafyllou, C. et al. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage 26, 243–250 (2005).
8. Kong, Y., Jenkinson, M., Andersson, J., Tracey, I. & Brooks, J. C. W. Assessment of physiological noise modelling methods for functional imaging of the spinal cord. NeuroImage 60, 1538–1549 (2012).
9. Brooks, J. C. W. et al. Physiological noise modelling for spinal functional magnetic resonance imaging studies. NeuroImage 39, 680–692 (2008).
10. Zhang, B., Seifert, A. C., Kim, J.-W., Borrello, J. & Xu, J. 7 Tesla 22-channel wrap-around coil array for cervical spinal cord and brainstem imaging. Magn. Reson. Med. 78, 1623–1634 (2017).
11. Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med Image Anal 5, 143–156 (2001).
12. De Leener, B. et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. NeuroImage 145, 24–43 (2017).
13. Woolrich, M. W., Ripley, B. D., Brady, M. & Smith, S. M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14, 1370–1386 (2001).
14. Shmueli, K. et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage 38, 306–320 (2007).
15. Birn, R. M., Diamond, J. B., Smith, M. A. & Bandettini, P. A. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–1548 (2006).
Figures