1459
Characterization of the spinal cord fMRI signal in the healthy subjects and in multiple sclerosis patients1CNR- Nanotec, Roma, Italy, 2CNR- Nanotec, Lecce, Italy, 3Enrico Fermi Reserch Centre, Rome, Italy, 4CREF-Centro Fermi, Roma, Italy, 5campus biomedico, Roma, Italy, 6fondazione Santa Lucia, Roma, Italy
Synopsis
Functional Magnetic Resonance Imaging (fMRI) has become one of the most powerful tools in neuroscience research, with promising applications in clinical practice. Particularly, fMRI based on blood oxygenation level dependent (BOLD) contrast has gained a primary role in the study of human brain and spinal cord, for the characterization of normal and pathological brain/spinal cord (SC) activity. In this framework, we have studied and report the characterization of the functional response of the SC to a multilevel motor task, designed to assess the linearity of the hemodynamic response as a function of the intensity of a graded task.
Introduction
Functional Magnetic Resonance Imaging techniques based on the Blood Oxygenation Level-Dependent (BOLD) signal are well established for the indirect study of the neuronal activity in the brain and spinal cord. Recently, studies on the task-dependent modulation of the SC fMRI activations signal in response to innocuous and painful sensory stimuli or motor tasks have been performed, and an hemodynamic response function (hrf) specific for the SC has been proposed1. It has been shown that there is a linear relationship between the applied force and the BOLD signal amplitude during isometric exercis2, and that there is also a movement rate-dependent increase in spinal fMRI signals3. All these studies have shown that spinal BOLD fMRI has the potential for becoming a reliable and sensitive tool for studying the modulation of functional activity in the SC. SC fMRI may be of immediate application in neuroradiology, because a non-invasive tool capable of monitoring the function, and thus complementing the available anatomical information, is a crucial need in these fields. Preliminary studies of people with spinal cord injuries and multiple sclerosis (MS) have demonstrated an altered activity in the SC, depending on the injury severity or the advancement of the disease4-8. In this framework we study and report the characterization of the functional response of the human SC to a multilevel motor task, designed to assess the linearity of the hemodynamic response as a function of the intensity of a graded task in healthy subjects and in multiple sclerosis patients.Methods
Acquisitions were performed on 45 healthy subjects, and 22 patients with multiple sclerosis employing a Philips Achieva 3 T MR scanner (Philips Medical Systems, Best, The Netherlands), equipped with a neurovascular coil array. fMRI data were acquired using a GRE-EPI sequence along axial directions, with the following parameters: TE/TR = 25/3000 ms, Flip angle = 80°, FOV = 140x140x143 mm3, acquisition matrix = 96x96x34, resolution giving a voxel size of= 1.5x1.5x3 mm3. Order of axial runs was randomized between subjects. Anatomical reference images were acquired using 3D T1-weighted gradient echo sequence (TE/TR = 5.89/9.59 ms, flip angle = 9°, FOV = 240x240x192 mm3, resolution = 0.75x0.75x1.5mm3). During all functional runs, Heart beat and pulse and respiration data were recorded using scanner integrated plethysmograph and respiratory belt during all functional runs. The acquisition protocol consisted in five epochs, each of them divided in task execution and resting state. Task execution requested to apply a given level of force, randomly selected among 20%, 40% or 50% of the total maximum sustainable voluntary contraction force (MSF), to the stimulation device. Immediately before the fMRI session, subjects underwent a training phase with the stimulation device outside the MR scanner. In a first trial, the MSF was determined. Subject were asked to press the device up to their maximum sustainable force, and to keep the force for 30s. Then, subjects were trained to perform the task. We also implemented and optimized a scfMRI preprocessing and data analysis pipeline, built around the Spinal Cord Toolbox (SCT)9. The statistical analysis approaches to study the linear relationship, is based on a standard SC hemodynamic function response1.Results & Discussion
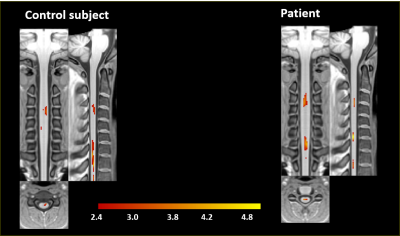
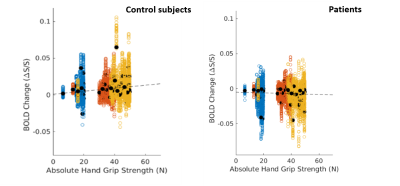
In figure 1 we compare the activation maps in the PAM50 template space of a control with respect to a patient. The active voxels in axial images are more consistent with grey matter activation. The longitudinal level of the activation is C3/C4. As expected, the functional activation is only found in ipsilateral spinal cord. We found that the activation is more intense in the patients. In figure 2 we report the mean BOLD change as a function of the grip strength in the patients and in the control subjects. The results obtained suggest a non parametric dependence of functional response in the spinal cord on the stimulation strength in an isometric motor task in the case of the patientsConclusions
In the case of patients, by studying the BOLD signal as a function of force, a statistically linear trend of activation with respect to force was not obtained: their ratio is practically constant. Overall, the present work provides an optimized methodological tool for advancing fMRI applied to the spinal cord in basic research, and towards applications in clinical practiceAcknowledgements
The FISR Project “Tecnopolo di nanotecnologia e fotonica per la medicina di precisione” (funded by MIUR/CNR, CUP B83B17000010001), the TECNOMED project(funded by Regione Puglia, CUP B84I18000540002).References
1. Giulietti, G., et al., Neuroimage, 2008. 42(2): p. 626-634.
2. Madi, S., et al., American journal of neuroradiology, 2001. 22(9): p. 1768-1774.
3. Maieron, M., et al., The Journal of neuroscience, 2007. 27(15): p. 4182-4190.
4. Agosta, F., et al., Neuroimage, 2008. 39(4): p. 1542-1548.
5. Agosta, F., et al., Magnetic Resonance in Medicine, 2008. 59(5): p. 1035-1042.
6. Kornelsen, J. and P. Stroman. Spinal Cord, 2007. 45(7): p. 485-490.
7. Valsasina, P., et al., Journal of Neurology, Neurosurgery & Psychiatry, 2010. 81(4): p. 405-408. 162
8. Wheeler-Kingshott, C., et al., Neuroimage, 2014. 84: p. 1082-1093.
9.De Leener B, Levy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D et al. Neuroimage 2017; 145(Pt A): 24-43.
Figures