1452
Thalamic Involvement in the Pediatric Chronic Spinal Cord Injured Population with Respect to the Severity of Injury1Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
Little is known about microstructural alterations in the thalamus following spinal cord injury (SCI), especially in the pediatric population. In this study, we used diffusion tensor imaging (DTI) derived metrics to characterize microstructural changes in thalamic nuclei of pediatric subjects with chronic SCIs with respect to the severity of injury based on the American Spinal Injury Association Impairment Scale (AIS). We report significant differences in mean diffusion metrics in thalamic nuclei that are suggestive of microstructural alterations between different AIS classifications.
Introduction
Spinal Cord Injury (SCI) is a dynamic and progressive condition that has been shown to cause changes in the brain resulting from the effects of nerve deafferentation1. Prior literature has shown that the thalamus exhibits both structural and functional changes following SCI2-6, but little is known about any microstructural alterations. Additionally, little is known about these structural changes in the pediatric population. In this study, we used diffusion tensor imaging (DTI) derived metrics to characterize microstructural changes in thalamic nuclei in SCI patients.Methods
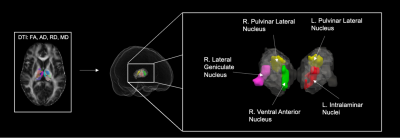
We conducted a retrospective study of 18 subjects aged 8-20 years old with chronic SCIs. Subjects were assigned to three groups after assessing for SCI severity via the American Spinal Injury Association Impairment Scale (AIS)7. Five subjects were classified as AIS grade A, nine subjects were classified as AIS grade B, and four subjects were classified as AIS grade C and D. The AIS C and D group were amalgamated due to limited subject number. DTI scans were acquired in a 3T Siemens Verio MR scanner. Initial raw diffusion data was corrected for motion artifacts and eddy current distortions. Then, diffusion tensor estimates per voxel were fit to generate the DTI scalars fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). These measures were normalized via non-linear registration to MNI space. Registration was performed using the Symmetric Normalization (SyN) transformation model via successively running SyN Quick and SyN8. Diffusion values were acquired with the AAL3 thalamic atlas for 15 major thalamic nuclei, and a multiple Mann-Whitney U test was applied to identify group differences with significance threshold set at p < 0.05.Results
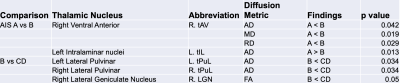
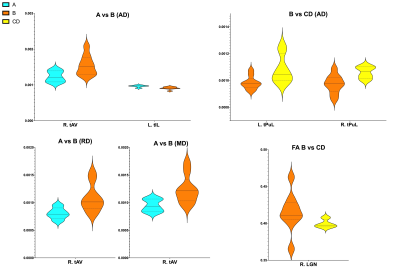
Mean DTI metrics in were significantly different in thalamic nuclei between AIS groups A vs B and B vs CD. Between AIS A vs B, AIS A demonstrated decreased AD, RD, and MD in the right ventral anterior nucleus (R. tAV) but increased AD in the left intralaminar nuclei group (L. tIL). Between B vs CD, AIS B demonstrated decreased FA in the right lateral geniculate nucleus (R. LGN) and decreased AD in both the left and right lateral pulvinar nuclei (tPuL). The results are displayed in figure 1 and displayed graphically in figure 2.Discussion
Our results indicate the presence of microstructural alterations in a few thalamic nuclei following SCI with respect to the severity based on AIS classification. These changes suggest the presence of microstructural alterations in thalamic nuclei in pediatric subjects with SCI. Furthermore, the changes in FA, AD, RD, and MD may indicate a variety of processes such as changes in myelination status or gliosis9,10. However, the diffusion metrics are not specific enough to elucidate an exact process. Nonetheless, the presence of these changes in certain major thalamic nuclei could correlate with clinical findings often observed with chronic SCI such as depression and pain11-13, and may further characterize the localization of structural changes occurring in the brain following SCI.Conclusion
DTI derived diffusion metrics may be useful as imaging biomarkers to localize possible neuroplastic changes in the brain after SCI and provide insight into how they may affect the thalamus’ role in sensorimotor and cognitive functions. While only few thalamic nuclei showed significant changes in diffusion values between AIS groups, these preliminary results are encouraging and warrant further studies with a larger population.Acknowledgements
No acknowledgement found.References
1. Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good? Neuroscience. 2014;283:78-94. doi:10.1016/j.neuroscience.2014.06.05618.
2. Alonso-Calviño E, Martínez-Camero I, Fernández-López E, Humanes-Valera D, Foffani G, Aguilar J. Increased responses in the somatosensory thalamus immediately after spinal cord injury. Neurobiol Dis. 2016;87:39-49. doi:10.1016/j.nbd.2015.12.00319.
3. Osinski T, Acapo S, Bensmail D, Bouhassira D, Martinez V. Central Nervous System Reorganization and Pain After Spinal Cord Injury: Possible Targets for Physical Therapy-A Systematic Review of Neuroimaging Studies. Phys Ther. 2020;100(6):946-962. doi:10.1093/ptj/pzaa04320.
4. Hawasli AH, Rutlin J, Roland JL, et al. Spinal Cord Injury Disrupts Resting-State Networks in the Human Brain. J Neurotrauma. 2018;35(6):864-873. doi:10.1089/neu.2017.521221.
5. Karunakaran KD, Yuan R, He J, et al. Resting-State Functional Connectivity of the Thalamus in Complete Spinal Cord Injury. Neurorehabil Neural Repair. 2020;34(2):122-133. doi:10.1177/154596831989329922.
6. Huber E, Patel R, Hupp M, Weiskopf N, Chakravarty MM, Freund P. Extrapyramidal plasticity predicts recovery after spinal cord injury. Sci Rep. 2020;10(1):14102. doi:10.1038/s41598-020-70805-5
7. Kirshblum SC, Biering-Sorensen F, Betz R, et al. International Standards for Neurological Classification of Spinal Cord Injury: cases with classification challenges. J Spinal Cord Med. 2014;37(2):120-127. doi:10.1179/2045772314Y.0000000196
8. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. doi:10.1016/j.media.2007.06.004 10.
9. Bellani M, Boschello F, Delvecchio G, et al. DTI and myelin plasticity in bipolar disorder: integrating neuroimaging and neuropathological findings. Front Psychiatry. 2016;7:21. doi:10.3389/fpsyt.2016.0002111.
10. Müller H-P, Roselli F, Rasche V, Kassubek J. Diffusion Tensor Imaging-Based Studies at the Group-Level Applied to Animal Models of Neurodegenerative Diseases. Front Neurosci. 2020;14:734. doi:10.3389/fnins.2020.0073434.
11. van Gorp S, Kessels AG, Joosten EA, van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain. 2015;19(1):5-14. doi:10.1002/ejp.52235.
12. Gibbs K, Beaufort A, Stein A, Leung TM, Sison C, Bloom O. Assessment of pain symptoms and quality of life using the International Spinal Cord Injury Data Sets in persons with chronic spinal cord injury. Spinal Cord Series and Cases. 2019;5:32. doi:10.1038/s41394-019-0178-836.
13. Williams R, Murray A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2015;96(1):133-140. doi:10.1016/j.apmr.2014.08.016
Figures