1451
Evaluating acquisition and preprocessing methods for diffusion MRI in the cervical spinal cord1Vanderbilt University Medical Center, Nashville, TN, United States, 2Vanderbilt University, Nashville, TN, United States
Synopsis
The aim of this study was to evaluate acquisition and preprocessing strategies for diffusion MRI in the cervical spinal cord. We tested 6 acquisition and 5 preprocessing strategies, and quantified evaluation criteria, and found that pipelines that include distortion correction significantly improve data quality. However, motion and lack of cardiac triggering significantly impact quality measures. Standard diffusion processing packages need to be adapted and modified for spinal cord microstructure to ensure accurate and robust diffusion quantification.
Introduction
Quantitative diffusion MRI (dMRI) is a promising tool to study the microstructure of the spinal cord (SC) in health and disease. However, in vivo SC dMRI is challenging due to the SC size and surrounding tissue interfaces, resulting in susceptibility to motion and distortion artifacts, while also requiring high spatial-resolution in a reasonable acquisition time. While dMRI acquisition and image pre-processing has largely focused, and been optimized for, brain imaging, there is much work needed to understand optimal pipelines for the spinal cord. Inspired by recent work evaluating distortion correction methods in the SC [1], we extend the analysis by evaluating combinations of acquisition (6 different acquisition types) and full preprocessing pipelines (5 pipelines) on signal-to-noise ratio, image contrast, and geometric fidelity. We further investigate robustness of these pipelines to subject motion, and the inclusion/exclusion of cardiac triggering.Methods
Data acquisitionThe scan cohort consisted of N=10 healthy volunteers (6F/4M, Age 29.5+/-8.7 years). All scanning was performed on a 3T Philips Ingenia. Acquisitions included a high-resolution multi-echo gradient echo (mFFE) (TR/TE/ΔTE=700/8.0/9.2ms, α=28 degrees, slices=14) for co-registration and to serve as a reference image. The diffusion sequences consisted of a cardiac-triggered spin echo with single-shot EPI readout (TR/TE=5 beats (~5000ms)/77 ms, resolution=1.1x1.1mm2, slice thickness=5mm, partial Fourier=0.693, slices=14, time ~4-6 minutes depending on heart rate). Images were centered between C3/C4. A single-shell acquisition was used with 32 diffusion-weighted directions at b=750s/mm2.
Datasets
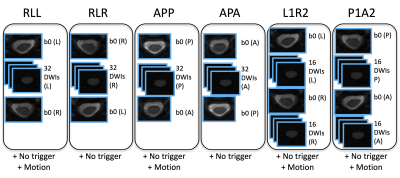
This sequence was repeated four times, with phase encoding (PE) in the right-left (RL) and anterior-posterior (AP) axis with fat-shift in left, right, posterior, and anterior (RLL, RLR, APP, APA, respectively). In addition to a typical acquisition (1 b=0 + 32 DWIs + 1 reverse PE b=0), we also generate ‘L1R2’ and ‘P1A2’ which is the first half of RLL with the second half of RLR, and the first half of APP with the second half of APA. In dMRI of the brain, there is evidence that collecting the full set of reverse DWIs in addition to the b0 is beneficial [2]. This generates 6 test sequences, all with 2 b=0 and 32 diffusion weighted images, shown in Figure 1.
Motion and cardiac triggering
Scanning was repeated without cardiac triggering (all other parameters constant) to assess the effects of triggering. Finally, the RLL and APP scans were repeated, asking the subject to move within the scan, in order to assess robustness to motion.
Distortion Correction
Pre-processing was done following 5 pipelines: (1) no corrections, with DTI fit voxel-wise to raw data directly; (2) motion-correction only, as implemented in the spinal cord toolbox (SCT) [3]; (3) HySCO correction [4] as part of the ACID toolbox; (4) TOPUP [5] as part of the FSL package; and (5) DR-BUDDI [6] as part of the TORTOISE package.
Evaluation
Acquisition and preprocessing were evaluated based on several criteria. In this abstract we focus on: (1) SNR of the b=0 image, (2) normalized mutual information (NMI) between the b=0 image and the mFFE as a measure of geometric fidelity, (3) NMI between the mean DWI (mDWI) and the mFFE in the cord only, (4) CNR between white and gray matter (WM, GM) in FA maps, (5) angular alignment between primary eigenvector and main cord axis, as proposed in [1] as a measure of orientation-correction.
Results
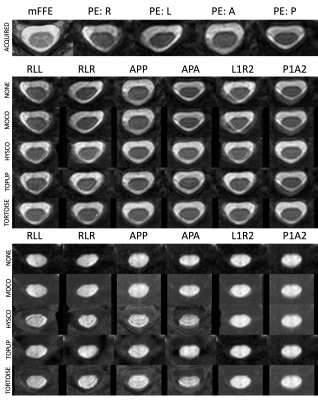
Qualitative ResultsQualitative results are shown in Figure 2, including the raw distorted data for all PE directions, and the corrected b=0 images and mDWI images for all combinations of acquisition and preprocessing. Visually, methods that correct distortion (HySCO, TOPUP, TORTOISE) better match the shape/geometry of mFFE, although boundary and noise artifacts are apparent in the CSF regions of the mDWI images.
Quantitative results
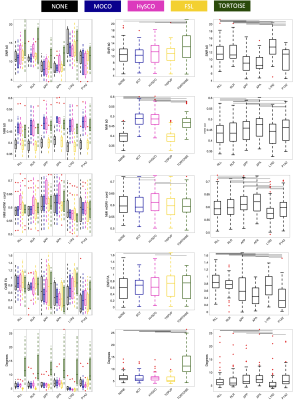
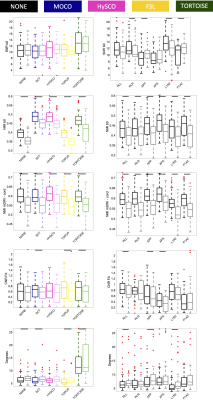
Figure 3 shows quantitative results of all evaluation measures, for all acquisitions and pipelines. The main takeaway is that all methods do well in different evaluation criteria, and none consistently outperform others. For example, TORTOISE results in higher SNR with high b=0 NMI, but results in angular misalignment and moderate NMI within the cord, whereas TOPUP has higher CNR and alignment, but poorer NMI with b=0. Differences are observed in acquisition as well, with R or L PE directions offering higher SNR and CNR, but lower alignment. Little advantages are observed in acquiring full sets of DWI with reverse PE.
Motion
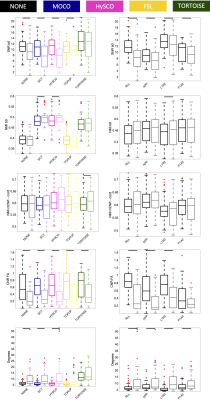
Results for motion-corrupted data are shown in Figure 4. Notably, motion-correction alone (SCT) is not enough for full correction, and pipelines suffer in all quality metrics, although TORTOISE is not as significantly impacted for many measures.
Triggering
Removing triggering results in significant reductions in all quality control metrics (Figure 5), for all acquisitions, except for the SNR of the b=0 image.
Discussion
We evaluated several acquisition strategies and several processing pipelines for dMRI in the spinal cord. In general, we find there is no optimal acquisition and preprocessing strategy, although we recommend the use of distortion correction methods in addition to motion correction. Significant innovations or adaptations are needed for standard diffusion processing toolboxes for spinal cord data, as has been done in the SCT [3, 7-10]. Assumptions of motion, fields, and contrasts may differ from that in the brain for which these are largely designed for.Acknowledgements
This work was supported by the National Science Foundation Career Award #1452485, the National Institutes of Health under award numbers R01EB017230, and in part by ViSE/VICTR VR3029 and the National Center for Research Resources, Grant UL1 RR024975–01, R01 EY023240, R01EB017230, and K01 K01EB030039.References
1. Snoussi, H., et al., Evaluation of distortion correction methods in diffusion MRI of the spinal cord. arXiv preprint arXiv:2108.03817, 2021.
2. Irfanoglu, M.O., et al., Improved reproducibility of diffusion MRI of the human brain with a four-way blip-up and down phase-encoding acquisition approach. Magn Reson Med, 2021. 85(5): p. 2696-2708. 3. De Leener, B., et al., SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage, 2017. 145(Pt A): p. 24-43.
4. Ruthotto, L., et al., Diffeomorphic susceptibility artifact correction of diffusion-weighted magnetic resonance images. Phys Med Biol, 2012. 57(18): p. 5715-31.
5. Andersson, J.L., S. Skare, and J. Ashburner, How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 2003. 20(2): p. 870-88.
6. Irfanoglu, M.O., et al., DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage, 2015. 106: p. 284-99.
7. Cohen-Adad, J., et al., Generic acquisition protocol for quantitative MRI of the spinal cord. Nat Protoc, 2021.
8. De Leener, B., et al., PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage, 2018. 165: p. 170-179.
9. De Leener, B., et al., Topologically preserving straightening of spinal cord MRI. J Magn Reson Imaging, 2017. 46(4): p. 1209-1219.
10. Dupont, S.M., et al., Fully-integrated framework for the segmentation and registration of the spinal cord white and gray matter. Neuroimage, 2017. 150: p. 358-372.
Figures