1448
Performance characterization of three coils for whole brain and/or cervical spinal cord MRI at 7T1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Institute of Medical Physics and Radiation Protection, University of Applied Sciences Mittelhessen, Giessen, Germany, 3NeuroPoly Lab, Institute of Biomedical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 4Centre for Functional and Metabolic Mapping, The University of Western Ontario, London, ON, Canada, 5Siemens Medical Solutions USA Inc., Malvern, PA, United States, 6Harvard-Massachusetts Institute of Technology Division of Health Sciences & Technology, Cambridge, MA, United States, 7Functional Neuroimaging Unit, CRIUGM, Université de Montréal, Montreal, QC, Canada, 8Mila – Quebec AI Institute, Montreal, QC, Canada
Synopsis
Several neurological diseases affect both the brain and spinal cord. 7T MRI enables better imaging of the narrow cord as well as single-subject prediction using fMRI. However, concurrent brain-cord 7T imaging requires a coil with good SNR and acceleration capabilities in both head and neck, which has been unavailable. To overcome this, we performed flip-angle, SNR, g-factor and tSNR characterization of a custom-built 16Tx/64Rx head/neck radiofrequency coil. We also compared it against a custom-built 8Tx/20Rx cervical spine coil as well as a commercial 8Tx/32Rx brain coil. This hardware will pave the way for concurrent brain-cord 7T MRI in the future.
Introduction
Several diseases affect both the brain and spine (e.g., multiple sclerosis1, chronic pain2, ALS3, Alzheimer’s disease4), and their concurrent imaging enables the study of brain-cord MRI, including functional MRI (fMRI) connectivity impairments5. Brain-cord fMRI has been demonstrated at 3T6–11 but not at 7T, although it can greatly benefit from 7T imaging12-14. The cord is narrow (~10mm) with dorsal gray-matter horns being <2mm wide. The field-of-view (FOV) of brain-cord imaging is also much larger. 7T imaging can enable high-SNR, high-sensitivity, high-spatiotemporal resolution, full-FOV brain-cord MRI, if certain challenges are overcome (higher B0 inhomogeneities, physiological noise and gradient demands). But so far there has been no 7T radiofrequency (RF) coil that can achieve this. Here, we characterize the performance of a recently-developed custom-built head/neck coil15, and compare it with a spine-only and a brain-only coil.Methods
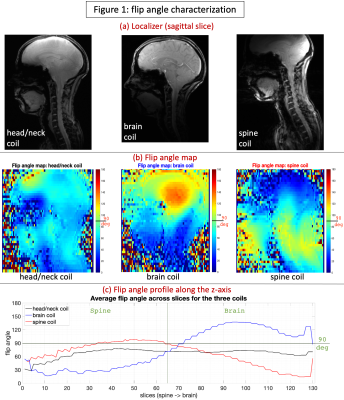
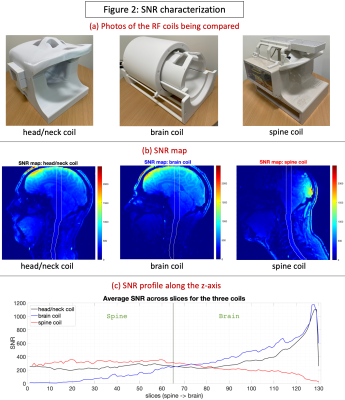
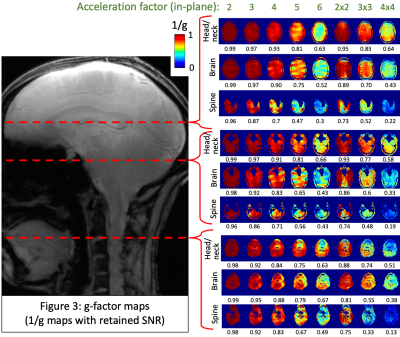
The custom-built 16-pTx 64-Rx head/neck coil15 consists of an anatomically shaped coil former to accommodate majority of adult heads (96th percentile). The housing was subdivided into an anterior and posterior portion. The array coil comprises 16 transmit (Tx) channels and 64 receive (Rx) elements. The Tx/Rx structures were merged into one housing instead of implementing a commonly-used separate tubular housing for the Tx. It was validated with both bench-level metrics (Q-factors, tuning, matching, inter-element coupling, active detuning efficiency) and safety metrics on the 7T scanner (RF heating, gradient heating, B1+).The coil was tested using the prototype pTx research mode of a 7T MAGNETOM Terra scanner (Siemens Healthcare, Erlangen, Germany). The study was approved by the Mass General Brigham IRB; informed consent was provided by the single participant. Flip angle (FA) maps were obtained using a vendor-provided turbo-flash (TFL) sequence that derives FA from gradient-echo (GRE) images acquired with and without a preceding saturation pulse (TR=16230ms, TE=2.07ms, FOV=256mm, voxel size=3.2×3.2×3mm3, slices=45, bandwidth=490Hz/px, reference amplitude=500V, B1 shim mode=TrueForm). A manually-drawn region-of-interest (ROI) along the z-axis was created (shown in Fig.2b) and mean per-slice FA was obtained for each coil. SNR maps were obtained from a reference GRE scan using an optimal coil combination weighted by the inverse of the noise covariance matrix16 (TR=8000ms, TE=3.82ms, FOV=256mm, voxel size=1×1×2mm3, slices=130, FA=78o, bandwidth=340Hz/px, reference amplitude=500V/0V). The optimal SNR coil combination was then divided by the sine of FA, which is important for isolating the receive sensitivity so that the SNR map is not a combination of transmit and receive efficiencies17. Parallel imaging geometry factor (g-factor) maps were computed from the reference GRE images18. To compare against benchmarks, these sequences were repeated with two other coils (shown in Fig.2a): (i) a custom-built second-generation 8-pTx 20-Rx cervical spine coil19, and (ii) a prototype 8-pTx 32-Rx brain coil (Nova Medical, Wilmington, MA, USA).
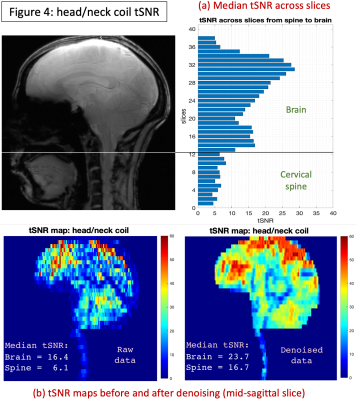
FMRI data was acquired using a prototype 2D echo-planar imaging sequence only with the head/neck coil: TR=1100ms, TE=18ms, FOV=180mm, voxel size=1.17×1.17×3mm3, slices=51, FA=60o, 4min acquisition, in-plane acceleration=3 (FLEET-DPG GRAPPA20,21), slice-acceleration=3 (simultaneous multi-slice22), bandwidth=2030Hz/px, reference amplitude=550V. FMRI data was manually separated into brain and spine parts and pre-processed using separate tools. Brain: data was pre-processed using CONN23 – motion correction, co-registration to MNI space, denoising (white-matter signal, CSF signal, motion parameters and their derivatives). Spine: data was pre-processed using in-house MATLAB- and AFNI-based tools24 – not-cord denoising, motion correction, co-registration and manual tissue segmentation (mean functional image), CSF and white-matter denoising. Temporal SNR (tSNR; ratio of mean to SD of the time series) was estimated for each voxel with both raw and fully-processed (denoised) data. Denoised tSNR is also important because higher tSNR gains can be made upon denoising only if physiological noise sources are accurately represented in the data.
Results
The head/neck coil achieved a more consistent FA, close to 90o at the 500V transmit voltage, across the brain and spine (Fig.1), while the spine coil was consistent only in the spine and subtentorial brain regions. With the brain coil, spine FA was on average too low, while it was too high in specific regions of the brain (can be optimized, however, for brain-only imaging). With SNR (Fig.2), the head/neck coil performed slightly inferior to the spine coil in the spine (mean values: SNRhead/neck-coil=246.9, SNRbrain-coil=90.9, SNRspine-coil=321.9), and brain coil in the brain (SNRhead/neck-coil=400.1, SNRbrain-coil=496.6, SNRspine-coil=197.3). The head/neck coil showed superior g-factor across the brain and spine across different accelerations (Fig.3). FMRI tSNR was higher in the brain, as expected, and saw improvement after denoising (Fig.4).Discussion
We evaluated the Tx/Rx performance of the custom-built head/neck coil with the goal of enabling 7T brain-cord fMRI in the future. We found that, across the brain and spine, it provides consistent and satisfactory excitation (Fig.1), SNR (Fig.2) and g-factors (Fig.3), along with reasonable tSNR in a highly accelerated, high spatiotemporal resolution and large FOV fMRI acquisition (Fig.4). It performed much better than the brain coil in the spine and spine coil in the brain, and in general, only slightly inferior to the spine coil in the spine and brain coil in the brain. Although the spine coil is best suited for cervical spine imaging, only the head/neck coil will enable applications with concurrent brain-cord fMRI at 7T.Conclusion
This novel head/neck coil will enable concurrent brain–spinal cord MRI measurements at 7T.Acknowledgements
R.L.B. received funding from the U.S. National Institute of Biomedical Imaging and Bioengineering [R01EB027779]. L.L.W. received funding from the U.S. National Institutes of Health (NIH) [S10OD023637]. J.C.A. received funding from the Canada Research Chair in Quantitative Magnetic Resonance Imaging [950-230815], the Canadian Institute of Health Research [CIHR FDN-143263], the Canada Foundation for Innovation [32454, 34824], the Fonds de Recherche du Québec - Santé [28826], the Natural Sciences and Engineering Research Council of Canada [RGPIN-2019-07244], the Canada First Research Excellence Fund (IVADO and TransMedTech), the Courtois NeuroMod project, and the Quebec BioImaging Network [5886, 35450]. K.M.G. received funding from the Canada Foundation for Innovation, Canada First Research Excellence Fund to BrainsCAN, and the Brain Canada Platform Support Grant. We thank Dr. Jonathan Polimeni, MGH, for providing us certain MATLAB code used in this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. B. N. Conrad, R. L. Barry, B. P. Rogers, S. Maki, A. Mishra, S. Thukral, S. Sriram, A. Bhatia, S. Pawate, J. C. Gore and S. A. Smith, "Multiple sclerosis lesions affect intrinsic functional connectivity of the spinal cord.," Brain, vol. 141, no. 6, p. 1650–1664, 2018.
2. D. Reckziegel, E. Vachon-Presseau, B. Petre, T. J. Schnitzer, M. N. Baliki and A. V. Apkarian, "Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain," Pain, vol. 160, no. Suppl 1, p. S37‐S48, 2019.
3. M. de Albuquerque, L. M. Branco, T. J. Rezende, H. M. de Andrade, A. Nucci and M. C. França, "Longitudinal evaluation of cerebral and spinal cord damage in Amyotrophic Lateral Sclerosis," Neuroimage: Clinical, vol. 14, p. 269‐276, 2017.
4. R. M. Lorenzi, F. Palesi, G. Castellazzi, P. Vitali, N. Anzalone, S. Bernini, M. Cotta Ramusino, E. Sinforiani, G. Micieli, A. Costa, E. D'Angelo and C. Gandini Wheeler-Kingshott, "Unsuspected Involvement of Spinal Cord in Alzheimer Disease.," Frontiers in cellular neuroscience, vol. 14, p. 6, 2020.
5. J. Zhang, A. Kucyi, J. Raya, A. N. Nielsen, J. S. Nomi, J. S. Damoiseaux, D. J. Greene, S. G. Horovitz, L. Q. Uddin and S. Whitfield-Gabrieli, "What have we really learned from functional connectivity in clinical populations?," NeuroImage, vol. 242, p. 118466, 2021.
6. J. Cohen-Adad, C. J. Gauthier, J. C. Brooks, M. Slessarev, J. Han, J. A. Fisher, S. Rossignol and R. D. Hoge, "BOLD signal responses to controlled hypercapnia in human spinal cord.," NeuroImage, vol. 50, no. 3, p. 1074–1084, 2010.
7. J. Finsterbusch, C. Sprenger and C. Büchel, "Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update.," NeuroImage, vol. 79, p. 153–161, 2013.
8. S. Vahdat, O. Lungu, J. Cohen-Adad, V. Marchand-Pauvert, H. Benali and J. Doyon, "Simultaneous Brain-Cervical Cord fMRI Reveals Intrinsic Spinal Cord Plasticity during Motor Sequence Learning.," PLoS biology, vol. 13, no. 6, p. e1002186, 2015.
9. H. Islam, C. Law, K. A. Weber, S. C. Mackey and G. H. Glover, "Dynamic per slice shimming for simultaneous brain and spinal cord fMRI.," Magnetic resonance in medicine, vol. 81, no. 2, p. 825–838, 2019.
10. A. Tinnermann, S. Geuter, C. Sprenger, J. Finsterbusch and C. Büchel, "Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia.," Science, vol. 358, no. 6359, p. 105–108, 2017.
11. S. Vahdat, A. Khatibi, O. Lungu, J. Finsterbusch, C. Büchel, J. Cohen-Adad, V. Marchand-Pauvert and J. Doyon, "Resting-state brain and spinal cord networks in humans are functionally integrated.," PLoS biology, vol. 18, no. 7, p. e3000789, 2020.
12. R. L. Barry, S. J. Vannesjo, S. By, J. C. Gore and S. A. Smith, "Spinal cord MRI at 7T.," NeuroImage, vol. 168, pp. 437-451, 2018.
13. N. Bruschi, G. Boffa and M. Inglese, "Ultra-high-field 7-T MRI in multiple sclerosis and other demyelinating diseases: from pathology to clinical practice.," European radiology experimental, vol. 4, no. 1, p. 59, 2020.
14. J. Abraham, "Imaging for head and neck cancer.," Surgical oncology clinics of North America, vol. 24, no. 3, p. 455–471, 2015.
15. M. W. May, S. L. J. D. Hansen, N. Kutscha, G. K. Multani, M. Mahmutovic, M. Poniatowski, R. Gumbrecht, R. Kimmlingen, M. Adriany, Y. Chang, B. Guerin, C. Triantafyllou, L. L. Wald and B. Keil, "A Patient-Friendly 16ch Tx / 64ch Rx Array for Combined Head and Neck Imaging at 7 Tesla.," in Annual Meeting of the International Society for Magnetic Resonance in Medicine, Virtual Meeting, 2021.
16. P. Kellman and E. R. McVeigh, "Image reconstruction in SNR units: a general method for SNR measurement.," Magnetic resonance in medicine, vol. 54, no. 6, p. 1439–1447, 2005.
17. K. Uğurbil, E. Auerbach, S. Moeller, A. Grant, X. Wu, P. F. Van de Moortele, C. Olman, L. DelaBarre, S. Schillak, J. Radder, R. Lagore and G. Adriany, "Brain imaging with improved acceleration and SNR at 7 Tesla obtained with 64-channel receive array.," Magnetic resonance in medicine, vol. 82, no. 1, p. 495–509, 2019.
18. K. P. Pruessmann, M. Weiger, M. B. Scheidegger and P. Boesiger, "SENSE: sensitivity encoding for fast MRI.," Magnetic resonance in medicine, vol. 42, no. 5, p. 952–962, 1999.
19. N. Lopez Rios, R. Topfer, A. Foias, A. Guittonneau, K. M. Gilbert, R. S. Menon, L. L. Wald, J. P. Stockmann and J. Cohen-Adad, "Integrated AC/DC coil and dipole Tx array for 7T MRI of the spinal cord," in Proceedings of the International Society for Magnetic Resonance in Medicine, Montréal, QC, Canada, 2019.
20. A. I. Blazejewska, H. Bhat, L. L. Wald and J. R. Polimeni, "Reduction of across-run variability of temporal SNR in accelerated EPI time-series data through FLEET-based robust autocalibration.," NeuroImage, vol. 152, p. 348–359, 2017.
21. W. S. Hoge and J. R. Polimeni, "Dual-polarity GRAPPA for simultaneous reconstruction and ghost correction of echo planar imaging data.," Magnetic resonance in medicine, vol. 76, no. 1, p. 32–44, 2016.
22. K. Setsompop, B. A. Gagoski, J. R. Polimeni, T. Witzel, V. J. Wedeen and L. L. Wald, "Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty.," Magnetic resonance in medicine, vol. 67, no. 5, p. 1210–1224, 2012.
23. S. Whitfield-Gabrieli and A. Nieto-Castanon, "Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks," Brain Connectivity, vol. 2, no. 3, pp. 125-141, 2012.
24. R. L. Barry, B. N. Conrad, S. A. Smith and J. C. Gore, "A practical protocol for measurements of spinal cord functional connectivity," Scientific Reports, vol. 8, no. 1, p. 16512, 2018.
Figures