1445
A novel 1H-dipole/31P-birdcage transceiver coil for head imaging at 7T1UMC Utrecht, Utrecht, Netherlands, 2Wavetronica B.V., Utrecht, Netherlands, 3University Medical Center Utrecht, Utrecht, Netherlands, 4Eindhoven University of Technology, Eindhoven, Netherlands, 5Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield, United Kingdom, 6Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
A novel coil design with a 31P birdcage and a 1H dipole array is presented for the purpose of fMRS applications. This design allows for free line-of-sight for fMRI and fMRS visual stimulation via mirrors and despite a lower fill-factor performs comparably to a smaller dual-tuned birdcage. The matching of both frequencies is adequate for a range of human head circumferences from 52cm through 57cm. The 31P birdcage performance is not significantly degraded by presence of 1H dipoles when compared to a conventional birdcage dual-tuning strategy with Foster networks.
Introduction
31P-MRS gives information about concentrations of key high-energy phosphate metabolites and their rate of creation and use in vivo. Changes in metabolism in the brain are implicated in neurological and psychiatric conditions.With the development of ultra-high field systems dynamic (functional) in vivo 31P spectroscopy(1) is practicable. Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique producing changes in cortical excitability and firing rates-markers of metabolic activity(2). In this work, a 31P head coil for 7T is developed to be compatible with tDCS equipment and functional brain activation via visual stimulation.
Unlike previous head coils, (3,4), for the purposes of image registration, B0 shimming and f0 determination, the simultaneous use of a 1H coil is imperative. This results in a penalty for dual-tuning in the range of 10%-30% of the SNR as compared to a single-tuned coil(5,6,7,8,9,10,11,12).
Methods
This 1H/31P head coil consists of a single-tuned 31P birdcage, with a larger-than-usual radius to accommodate mirrors for allowing visual stimuli for fMRI studies (Fig. 1). Taking advantage of symmetry and to minimise coupling, four meandered proton dipoles(13,14) are placed in line with the inner endring-edges but between the legs of the birdcage, and without overlapping with the end rings.As a comparison, a 1H/31P dual-tuned birdcage with diameter 24cm is tested with protocols identical to the novel coil.
Simulations
Simulations are first performed for both constituent coils in isolation before the combined setup is simulated (Fig. 2). The simulations are performed in a 2L tissue simulating phantom (σ=1.079S/m; μr=1; εr=78), and in male and female human body models (Duke and Ella, ITIS foundation, Zurich) with the software Sim4Life (Zurich Medtech).The effect of two coils on each other is quantified in terms of the difference in B1+ and the SAR values as compared to the single-frequency setups. The simulations for B1+ and 10g averaged pSAR are normalised to 1W of total input power. This significantly reduces the coupling to the other coil and by only using 4 dipoles, it is possible to preserve symmetry and thus homogeneity of the birdcage while also leaving the line-of-sight open for subjects.
Bench
To address the coupling between the dipoles and the birdcage, an iterative approach to tuning is used. First, the birdcage coil is built with capacitor values to tune it to within 10MHz of the target frequency (120.3MHz). The proton dipoles are then introduced and the phosphorus coil is re-tuned. Finally the dipoles and the birdcage are fine-tuned in the presence of a realistic load and human heads. The bench performance compared to a dual-tuned birdcage coil built in-house(15). Bench measurements made using a network analyser (Fig. 3.) give sufficient confidence in the suitability of the coil towards scanner testing. No decoupling circuitry is necessary (S21 of -8dB to -12dB).
Scanner
Phantom safety scans are performed before the first subject scans. The coil is located using a low SNR, fast localising protocol. Thereafter, the B0 and B1 shimming is performed so as to enable f0 determination as the inhomogeneity on the B field may cause the phosphorus spectra to become indeterminate. Identical B1 maps are acquired for both coils in phantoms and in vivo.The 31P performance is quantified by looking at the power needed to achieve a given flip-angle in comparison to a known reference coil. In this case, we step the flip-angle from 40 degrees in steps of 20 degrees to determine the peak of the sinusoidal envelope. Subsequently, the same protocol is repeated on the reference dual-tuned birdcage coil.
Results
See figure 2 for bench results1H
Simulations
With a simulated 10g average of 0.7W/Kg from simulations, a maximum input power of 3W/ channel can be used before local SAR exceeds 10 W/kg. With a safety factor of 2, average power is limited to 1.5W/channel.
Scanner
B1 maps have similar amplitude for both coils invivo and in the phantom (~12uT)- however, the shimmed dipole-array is more homogeneous than the dual-tuned birdcage
31P
Scanner
Both coils reach the 90 degree(ref) flip-angle at about the same flip-angle setting with all other parameters maintained.
Discussion
The presence of the 1H dipole array and 31P birdcage detunes both coils, which necessitates iterative re-tuning.While a similar 1H B1 of 12uT is reached in the phantom and in vivo with both, the novel and comparison coils, the dipole array shows a more homogeneous excitation due to phase-shimming on 4 channels. The 31P performance of both coils is also similar, with the smaller, dual-tuned coil outperforming the larger novel coil by a factor of 15% in a phantom.
Conclusion
Despite having a larger diameter than the reference coil, the novel coil achieves similar B1+ efficiency for both frequencies. This indicates inherently better decoupling between the two frequencies as a result of coil geometry. Shimming the four proton channels gives more homogeneous 1H excitation.The large gap between coil and shield facilitates the use of a mirror and thus the ability to provide visual cues to the subject with minimum interference to the symmetry of the coil. The relatively large coil size could also permit the use of EEG caps or tDCS (trans-cranial direct current stimulation16) for other neuro studies(17,18 )
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 801075, 'NICI'References
- Hendriks, A. D., van der Kemp, W. J. M., Luijten, P. R., Petridou, N., & Klomp, D. W. J. (2019). SNR optimized (31) P functional MRS to detect mitochondrial and extracellular pH change during visual stimulation. NMR in Biomedicine, 32(11), e4137. https://doi.org/10.1002/nbm.4137
- Stagg CJ, Nitsche MA. Physiological Basis of Transcranial Direct Current Stimulation. The Neuroscientist. 2011;17(1):37-53. doi: 10.1177/1073858410386614
- van Uden, MJ, Peeters, TH, Rijpma, A, Rodgers, CT, Heerschap, A, Scheenen, TWJ. An 8-channel receive array for improved 31P MRSI of the whole brain at 3T. Magn Reson Med. 2019; 82: 825– 832. https://doi.org/10.1002/mrm.27736
- Rowland BC, Driver ID, Tachrount M, Klomp DWJ, Rivera D, Forner R, et al. Whole brain 31P MRSI at 7T with a dual-tuned receive array. Magn Reson Med. (2020) 83:765–75. doi: 10.1002/mrm.27953
- Matson, G.B., Vermathen, P. and Hill, T.C. (1999), A practical double-tuned 1H/31P quadrature birdcage headcoil optimized for 31P operation. Magn. Reson. Med., 42: 173-182. https://doi.org/10.1002/(SICI)1522-2594(199907)42:1<173::AID-MRM23>3.0.CO;2-O
- Murphy-Boesch, J. (2011). Double-Tuned Birdcage Coils: Construction and Tuning. In eMagRes (eds R.K. Harris and R.L. Wasylishen). https://doi.org/10.1002/9780470034590.emrstm1121
- Gamaliel Isaac, Mitchell D Schnall, Robert E Lenkinski, Katherine Vogele, A design for a double-tuned birdcage coil for use in an integrated MRI/MRS examination, Journal of Magnetic Resonance (1969), Volume 89, Issue 1, 1990, Pages 41-50, ISSN 0022-2364, https://doi.org/10.1016/0022-2364(90)90160-
- B.Lykowsky G, Carinci F, Düring M, Weber D, Jakob PM, Haddad D. Optimization and comparison of two practical dual-tuned birdcage configurations for quantitative assessment of articular cartilage with sodium magnetic resonance imaging. Quant Imaging Med Surg. 2015 Dec;5(6):799-805. doi: 10.3978/j.issn.2223-4292.2015.11.06.
9. Potter W, Wang L, McCully K, Zhao Q. Evaluation of a New 1H/31P Dual-Tuned Birdcage Coil for 31P Spectroscopy. Concepts Magn Reson Part B Magn Reson Eng. 2013;43(3):90-99. doi:10.1002/cmr.b.21239
10. M. F. et al., "Double Tuned 1H-23Na Birdcage Coils for MRI at 7 T : Performance evaluation through electromagnetic simulations," 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), 2018, pp. 1-6, doi: 10.1109/MeMeA.2018.8438777
11. Bart van de Bank, “Advancing technologies in 1H and 31P MR spectroscopy in the human brain at 7 Tesla”, PDF hosted at the Radboud Repository of the Radboud University Nijmegen, https://repository.ubn.ru.nl/handle/2066/182798
12. Gajawada, Gowtham, “Design and Development of Dual Tuned 19F and 1H RF Birdcage Coils for Small Animal MRI at 3T”, Lakehead University, KnowledgeCommons, https://knowledgecommons.lakeheadu.ca/handle/2453/752
13. Krikken, E, Steensma, BR, Voogt, IJ, et al. Homogeneous B1+ for bilateral breast imaging at 7 T using a five dipole transmit array merged with a high density receive loop array. NMR in Biomedicine. 2019; 32:e4039. https://doi.org/10.1002/nbm.4039Q.
14. Balzano, O. Garay and K. Siwiak, "The near field of dipole antennas, part II: Experimental results," in IEEE Transactions on Vehicular Technology, vol. 30, no. 4, pp. 175-181, Nov. 1981, doi: 10.1109/T-VT.1981.23902.
15. van der Kemp, Wybe J.M.; Klomp, Dennis W.J.; Wijnen, Jannie P. . 31P T2 of phosphomonoesters, phosphodiesters, and inorganic phosphate in the human brain at 7T. Magnetic Resonance in Medicine, (2017), –. doi:10.1002/mrm.27026
16. Bachtiar V, Near J, Johansen-Berg H, Stagg CJ. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife. 2015 Sep 18;4:e08789. doi: 10.7554/eLife.08789.
17. Navarro de Lara, L.I., Windischberger, C., Kuehne, A., Woletz, M., Sieg, J., Bestmann, S., Weiskopf, N., Strasser, B., Moser, E. and Laistler, E. (2015), A novel coil array for combined TMS/fMRI experiments at 3 T. Magn. Reson. Med., 74: 1492-1501. https://doi.org/10.1002/mrm.25535
18. Goetz SM, Deng ZD. The development and modelling of devices and paradigms for transcranial magnetic stimulation. Int Rev Psychiatry. 2017 Apr;29(2):115-145. doi: 10.1080/09540261.2017.1305949.
Figures
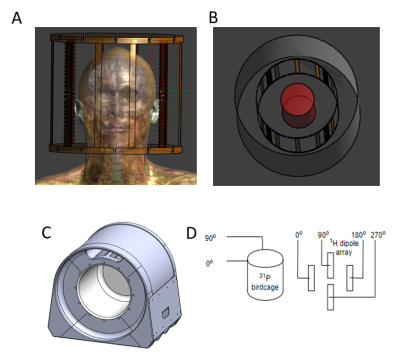
Figure 1:
Coil design:
A: 31P birdcage and dipole array with male body model
B: Coil loaded with phantom
C: Mechanical housing
D: Schematic of 31P birdcage and 1H dipole array
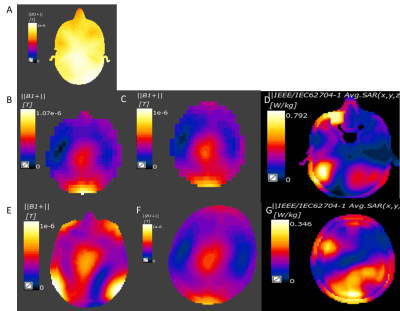
Figure 2: In vivo simulations in male and female body models
A: 31P birdcage only B1+ map in male body model
B:B1+ in 1H dipole array, male
C:B1+ in 1H dipole array, female
D:1H SAR 31P Birdcage with 1H dipoles, male
E:1H B1+ 31P Birdcage with 1H dipoles, male
F:1H B1+ 31P Birdcage with 1H dipoles, female
G:1H SAR 31P Birdcage with 1H dipoles, female

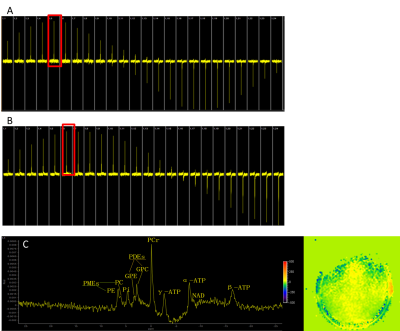
Figure 4:
31P birdcage performance evaluation
A: Flip-angle series (from 40 degrees in steps of 20 degrees) in reference dual-tuned birdcage coil: peak at 120 degrees
B: Flip-angle series (from 40 degrees in steps of 20 degrees) in new single tuned birdcage coil: peak at 140 degrees
C: 31P in vivo spectrum in female adult with shimmed B0+ map inset
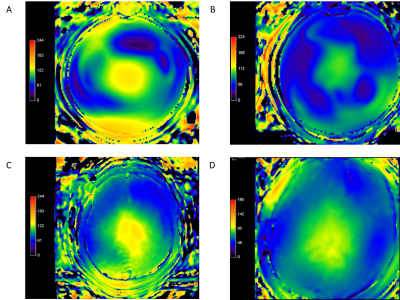
Figure 5:
1H coil performance characterisation with B1 maps. Both coils give ~12uT
A: Old dual-tuned 1H/31P birdcage with 0/90 fixed shim in phantom
B: New 1H dipole array shimmed with parallel transmit in phantom
C: Old dual-tuned 1H/31P birdcage with 0/90 fixed shim in female subject
D: New 1H dipole array shimmed with parallel transmit in female subject