1442
Influence of Glycosaminoglycans on the Transmetallation and Transchelation Kinetics of Gadolinium from GBCAs in the Presence of ZnCl21Deutsches Krebsforschungszentrum, Heidelberg, Germany, 2Physikalisch-Technische Bundesanstalt, Berlin, Germany, 3Charite, Berlin, Germany
Synopsis
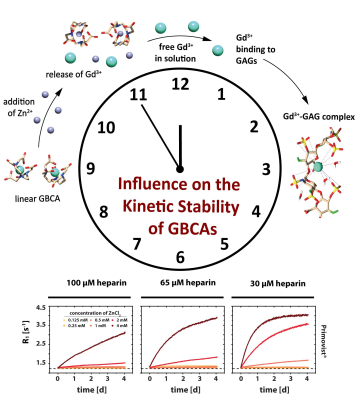
Relaxation rates in aqueous solutions containing 30-100 µM heparin as well as 150 µM GBCA were measured as a function of time. Different ZnCl2 stimuli with concentrations between 0.125-4 mM were used as competing ions that initiate a transmetallation and a transchelation process of the Gd3+ ion from GBCAs to glycosaminoglycans. The time resolved relaxometry measurements indicate that glycosaminoglycans play a concentration-dependent double role as competing chelator structures. They foster the thermodynamic instability of intact GBCA by sequestering Gd3+ from the contrast agent but simultaneously interact with competing ions and thus cause a reduced kinetic instability.
Introduction
Clinically used Gadolinium-based contrast agents (GBCA) require a high stability of the Gd3+ complex even in the presence of competing ions and/or chelators. However, the accumulation of Gd3+ ions in the human body1-3 was repeatedly observed on T1-weighted images after the administration of GBCA4-6. Insights about the exact mechanism of Gd3+ deposition in various tissues remain, however, elusive. Sugar structures like glycosaminoglycans (GAGs) are potential candidates to enlighten this missing piece2. This study aims to elucidate the influence of different heparin concentrations on the kinetic stability of GBCAs in the presence of different ZnCl2 concentrations.Methods
We studied solutions with different concentrations of heparin (0 µM-100 µM) and ZnCl2 (0.125-4 mM) in combination with five linear GBCAs (150 µM). All time resolved MR measurements were performed on a vertical 9.4 T MRI system. R1 measurements at 25 °C were performed using a dephasing recovery sequence. All R1 values reflect ROI-averaged values from corresponding R1 parameter maps.Results
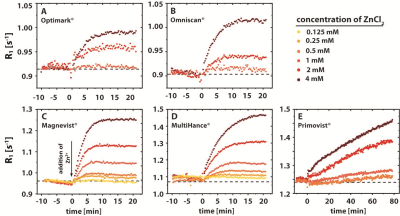
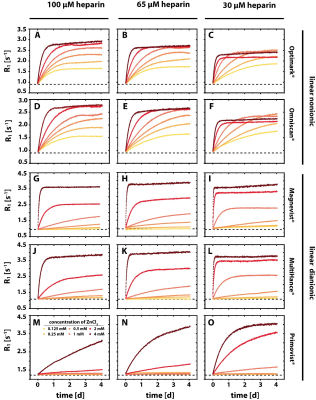
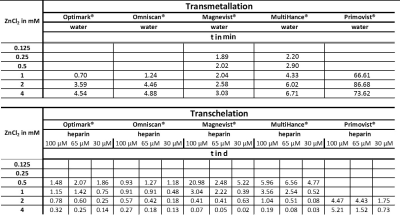
A release of Gd3+ from GBCAs that is triggered by Zn2+ ions can be observed via an increase in the relaxation rate. In this process, part of the low relaxivity contribution of the GBCAs is replaced with the higher relaxivity contribution of Gd3+ in water or from the Gd3+ GAG complexes7. The determined time constants for the transmetallation process vary significantly between the different GBCAs and for the investigated ZnCl2 concentrations (Tab.1). The final R1 values after the transmetallation (plateau values of the curves) increase with increasing ZnCl2 concentrations. A rather special behavior was observed for Primovist® where the data does not reach a stable plateau during the observation time window. The time constants for the transchelation process in the aqueous heparin solutions differ significantly from the time constants for the transmetallation process in water (Tab.1). While the time constants for the transmetallation process were in the order of 2 minutes and slightly increased with increasing ZnCl2 concentrations, the time constants for the transchelation are in the order of hours to weeks and tremendously decrease with increasing ZnCl2 concentrations.Discussion
Our investigations by time resolved MR relaxometry could show that the Zn/GAG ratio is critical to regain faster exchange kinetics during the transchelation and transmetallation process of Gd3+ from GBCAs. More Zn2+ ions potentially reduce the availability of binding sites in heparin. It was observed that the overall transchelation process is significantly more efficient for a high Zn/GAG ratio (Tab.1). The explanation could be that the larger ZnCl2 concentration causes on average more Zn2+ to be outside the GAG and thus encounter GBCA complexes with a subsequent quick transmetallation step. Most importantly, contrary to the relatively fast displacement of Gd3+ by Zn2+ in the absence of competing chelators, the overall transchelation of Gd3+ to heparin needs up to multiple days to achieve a new chemical equilibrium (Tab.1 and Fig.2). This reflects the complex situation when Gd3+ ions and other cations compete for both the binding in the parent GBCA chelator and the binding sites in the GAG. It could be concluded that for small ZnCl2 stimuli, the transmetallation is the limiting step and not the availability of binding sites for the subsequent chelation of dissociated Gd3+ to GAGs. However, from the transmetallation experiments in aqueous solutions, it was shown that the addition of ZnCl2 causes the release of Gd3+ ions within a few minutes (Tab.1 and Fig.1). Hence, the stability of the GBCA complex per se cannot be the limiting factor either. It is thus further concluded that the Zn2+ ions are sufficiently withheld by their own binding to heparin from initiating the transmetallation of the GBCA. For strong ZnCl2 stimuli, the time constant drops significantly. Due to the above-mentioned fact that Gd3+ outperforms Zn2+ in terms of binding to the GAG, the released Gd3+ ions are apparently not limited in finding a binding site and forming the macromolecular Gd-GAG complexes.Conclusion
We were able to demonstrate that GAGs as endogenous sugars play an important double role as competing chelators. Firstly, they foster the thermodynamic instability of intact GBCA in vitro by sequestering Gd3+ from the disfavored ZnL + Gd3+ intermediate. At the same time, the GAG’s interaction with competing ions can suppress the initial attack and reduce kinetic instability significantly. These findings add an important aspect to the ongoing discussion of gadolinium depositions in the human body after the application of GBCAs.Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Grant No. 289347353(GRK 2260) and Koselleck Grant No. 316693477 (SCHR 995/5–1). Support by the Dieter Morszeck Stiftung is also gratefully acknowledged.References
1) Laurent, S., Elst, L. V, Copoix, F. & Muller, R. N. Stability of MRI paramagnetic contrast media: a proton relaxometric protocol for transmetallation assessment. Invest. Radiol. 36, 115–22 (2001).
2) Taupitz, M. et al. Gadolinium-containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast Media Mol. Imaging 8, 108–16 (2013).
3) Gianolio, E., Gregorio, E. Di & Aime, S. Chemical Insights into the Issues of Gd Retention in the Brain and Other Tissues Upon the Administration of Gd-Containing MRI Contrast Agents. Eur. J. Inorg. Chem. 2019, 137–151 (2019).
4) Kanda, T., Ishii, K., Kawaguchi, H., Kitajima, K. & Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270, 834–41 (2014).
5) Kanda, T. et al. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 275, 803–9 (2015).
6) Radbruch, A. et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275, 783–91 (2015).
7) Werner, P., Taupitz, M., Schröder, L., & Schuenke, P. An NMR relaxometry approach for quantitative investigation of the transchelation of gadolinium ions from GBCAs to a competing macromolecular chelator. Scientific Reports, 11(1), 1-13 (2021).
Figures