1435
MRI based Radiomics for Distinguishing IDH-mutant from IDH wild-type Grade-4 Astrocytomas1Medical physics and biomedical engineering, Tehran university of medical science, Karaj, Iran (Islamic Republic of), 2Medical Imaging, University of Geneva, Geneva, Switzerland, 3Cardiovascular Medical and Research Center, Iran University of Medical Science, Tehran, Iran (Islamic Republic of), 4Hematology-Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 5Pathology and Lab Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 6Neurosurgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 7Radiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
Synopsis
To distinguish IDH-mutant from IDH wild-type grade-4 astrocytomas, conventional MR imaging (post-contrast T1-weighted and T2-Flair images) was acquired from 57 patients [IDH-mutant (n=23) and IDH-wild-type (n=34) grade-4 astrocytomas]. Post-contrast T1-weighted and T2-FLAIR images were resliced, resampled and co-registered. Neoplasms were segmented into whole tumor, enhancing region, central necrotic region, edema region, and core tumor (contrast enhancing + necrotic region). A total of 105 first-, second-, higher-order and shape based radiomics features were extracted from each ROI. Readily interpretable and quantitative features from different sub-regions of neoplasms were observed with high diagnostic performances in distinguishing IDH-mutant from IDH wild-type grade-4 astrocytomas.
Introduction
Mutations in isocitrate dehydrogenase (IDH) gene occurs in 70% of WHO grade II/III gliomas and in 10-15% of grade-4 astrocytomas.1Although incidence rates of IDH mutation in grade-4 astrocytomas is low, it is equally important to develop imaging biomarkers to discriminate their IDH profiles for planning appropriate treatment strategies and for prognostication of these patients.2 Using MR spectroscopy, some studies3,4 including from our group5 have identified lower-grade (WHO grade 2 and 3) gliomas harboring IDH mutation by detecting 2-hydroxyglutarate (2-HG). However, not all IDH-mutant gliomas show neomorphic activity of 2-HG production.6 Moreover, use of MR spectroscopy requires development of sophisticated pulse sequences and post-processing tools. Therefore, it is essential to develop conventional MR imaging-based biomarkers in distinguishing IDH-mutant from IDH-wild-type grade-4 astrocytomas. With this intent, the current study was designed to investigate the potential of radiomic features extracted from conventional MR images in differentiating IDH-mutant from IDH wild-type grade-4 astrocytomas. To address the issue of intratumor heterogeneity present in these malignant brain neoplasms, we examined various subregions of these neoplasms in distinguishing two genotypes using multiple machine learning algorithms.Methods
A cohort of 57 treatment naïve patients with IDH-mutant grade-4 astrocytomas (n=23) and IDH-wild-type grade-4 astrocytomas (n=34) underwent anatomical imaging on a 3T MR system with standard parameters. T2-FLAIR and post-contrast T1-weighted images were resliced, resampled and co-registered. As shown in Figures 1 and 2, regions of interest (ROIs) were drawn manually from contrast enhancing region, central necrotic region, and core tumor (solid + necrotic regions) on post-contrast T1 weighted images. Additionally, T2-FLAIR images were used to segment whole tumor, and peri-tumor edematous region in each case using a method described previously.7 A total of 105 first-order, and second-order texture features along with shape characteristics were extracted using image biomarker standardization initiative compliant PyRadiomics package from each ROI.8,9 These features include: first-order (18), shape (13), gray level dependence matrix (GLDM) (14), gray level size zone matrix (GLSZM) (16), gray level run length matrix (GLRLM) (16), gray level co-occurrence matrix (GLCM) (23), and neighboring gray tone difference matrix (NGTDM) (5). Altogether, 525 radiomics features were extracted from each image from 5 ROIs. Diverse feature selection algorithms including recursive feature elimination (RFE), minimum redundancy maximum relevance (MRMR), and Boruta were employed to select image features. Patients were randomly divided into two mutually exclusive training (70%) and testing (30%) sets. A total of 10 single and ensembled classifiers [Support vector machine (SVM), decision tree (DT), logistic regression (LR), K-nearest neighbors (KNN), gradient boosting (GB), random forest (RF), naive bayes (NB), multilayer perceptron (MLP), eXtreme gradient boosting (XGB), and linear discriminant analysis (LDA)] were used to distinguish two genotypes of grade-4 astrocytomas. The SMOTE algorithms and classifiers were employed using the mlr library10 in R version 4.0.4 (The R Foundation, Vienna, Austria). To differentiate two groups (IDH-mutant and IDH grade-4 astrocytomas), receiver operating characteristic (ROC) curve analyses were performed to determine area under the ROC curve (AUC), accuracy (ACC) and sensitivity (SEN) and hence, were used as metrics for performance.Results
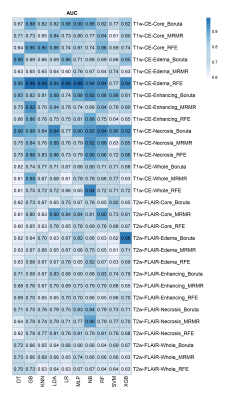
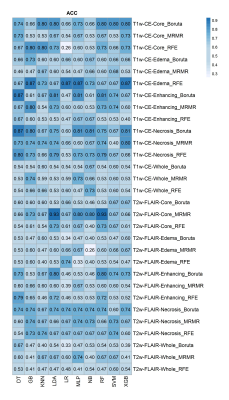
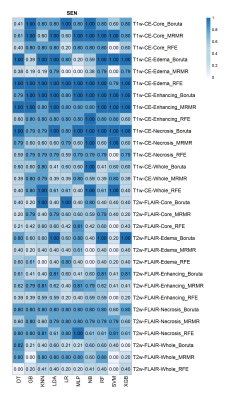
The highest accuracy for differentiating IDH-mutant from IDH wild type grade-4 astrocytomas was obtained from core tumor regions of neoplasms as visible on T2-Flair -images by using MRMR feature selection and LDA classifier (ACC=0.93, AUC = 0.9, SEN=0.79), and by using MRMR feature selection and RF classifier (ACC=0.93, AUC=0.9, SEN=0.79), followed by peri-tumor edematous regions of reregistered image with RFE feature selection and LR classifier (ACC=0.87, AUC =0.98, SEN=1). Additionally, the highest predive power (AUC) was obtained from edematous regions of reregistered image with RFE feature selection and LR classifier (AUC= 0.99). The AUC, ACC, and SEN heatmaps in differentiating IDH-mutant from grade-4 IDH wild-type grade-4 by using different selection features, and machine learning classifiers are shown in Figures 3-5 respectively.Discussion
Radiomics is an emerging translational field that automatically produces mineable high dimensionality data from MR images that can be used in diagnosis, classifying molecular profiles, predicting and evaluating treatment outcomes in patients with various brain neoplasms.11 Some earlier studies12,13 have identified specific radiomic features of neoplasms in distinguishing IDH-mutant grade-4 astrocytomas from IDH wild-type genotypes with variable accuracies. The strength of our study lies in the fact that we performed objective and high-throughput analysis of lesion textures using different feature selection and classification modules from different sub-regions of neoplasms as visible on T2-Flair and post-contrast T1 images. Our results indicate that readily interpretable and quantitative features can be extracted from a pre-defined ROIs encompassing solid, necrotic and peritumoral edematous regions of neoplasms in distinguishing IDH-mutant from IDH wild-type grade-4 astrocytomas with high accuracy. Limitations of this study include the retrospective nature of data analysis and the absence of external cohort validation.Conclusion
Our results revealed high diagnostic power of conventional MR imaging based radiomic features from different sub-regions of neoplasms in distinguishing IDH-mutant from IDH wild-type grade-4 astrocytomas. However, future studies with larger patient populations are required to validate our findings.Acknowledgements
This work was supported by funding obtained from University Research Foundation (URF), Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA (PI: Sanjeev Chawla, PhD).References
1. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773.
2. Yan W, Zhang W, You G, et al. Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PloS One. 2012;7(1):e30339.
3. Andronesi OC, Rapalino O, Gerstner E, et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013;123(9):3659-3663.
4. An Z, Tiwari V, Baxter J, et al. 3D high-resolution imaging of 2-hydroxyglutarate in glioma patients using DRAG-EPSI at 3T in vivo. Magn Reson Med. 2019;81(2):795-802.
5. Verma G, Mohan S, Nasrallah MP, et al. Non-invasive detection of 2-hydroxyglutarate in IDH-mutated gliomas using two-dimensional localized correlation spectroscopy (2D L-COSY) at 7 Tesla. J Transl Med. 2016;14(1):1-8.
6. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro-Oncol. 2009;11(4):341-347.
7. Bakas S, Reyes M, Jakab A, et al. Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. ArXiv Prepr ArXiv181102629. Published online 2018.
8. Van Griethuysen JJ, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104-e107.
9. Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative. ArXiv Prepr ArXiv161207003. 2016.
10. Bischl B, Lang M, Kotthoff L, et al. mlr: Machine Learning in R. J Mach Learn Res. 2016;17(1):5938-5942.
11. Rudie JD, Rauschecker AM, Bryan RN, et al. Emerging Applications of Artificial Intelligence in Neuro- Oncology. Radiology. 2019;290:607-618.
12. Tatekawa H, Hagiwara A, Uetani H, et al. Differentiating IDH status in human gliomas using machine learning and multiparametric MR/PET. Cancer Imaging. 2021;21(1):1-10.
13. Wang
D, Wang XY, Liu C, et al. Automated Machine-learning Framework Integrating Histopathological
and Radiological Information for Predicting IDH1 Mutation Status in Glioma. Front
Bioinforma. 2021; 52: 1-11.
Figures