1432
Towards a personalized MRgFUS treatment for tremor disorders: A study on the number of ablations using deep learning and structural connectivity1School of Computer Science, University of Sydney, Sydney, Australia, 2Brain and Mind Centre, University of Sydney, Sydney, Australia, 3Sydney Neuroimaging Analysis Centre, Sydney, Australia, 4St Vincent's Hospital Sydney, Sydney, Australia, 5GE Healthcare Australia, Melbourne, Australia
Synopsis
Disabling tremor is the most common symptom of tremor-dominated Parkinson’s disease (PD) patients. Recently, MR guided focused ultrasound (MRgFUS) has been applied in the clinical environment to treat tremor. However, patients can have different treatment responses to the ablation. Tremor suppression can be observed on some patients after ablating the ventral intermediate nucleus (Vim), while others require further ablations. To provide a tailored treatment, a deep learning technique is introduced in this work to predict whether a subject undergoing MRgFUS will respond to a single ablation in the VIM or require additional lesioning in other regions.
Introduction
MR-guided focused ultrasound (MRgFUS) is a non-invasive surgical procedure to treat tremor symptoms in tremor disorders, e.g. essential tremor (ET) and Parkinson’s Disease (PD). The aim is to ablate the tissue in regions implicated in tremor networks. Common targets of MRgFUS include the ventral intermediate nucleus (VIM), the posterior subthalamic area (PSA), and the ventral oral anterior nucleus (VOA)1. The optimal target choice is an active area of research and is often informed by the clinical presentation of tremor symptoms, and intra-procedural feedback following evaluation of tremor during the procedure. An accurate response prediction to a treatment target could assist clinicians in determining the optimal strategy. Given the irreversible nature of MRgFUS, this could help prevent avoidable tissue damage. Reducing the number of sonications required for an acceptable outcome could reduce side effects associated with edema2 and improve sonication efficiency, known to decrease over time due to heating effects3.To achieve this, we exploit the ability of deep learning approaches to encode meaningful features in a latent space. Our analysis was performed using pre-treatment structural connectomes labelled by the treatment targets chosen by the neurosurgeon. Additionally, we aim to reveal which regions and connections were most important in the model prediction.Methods
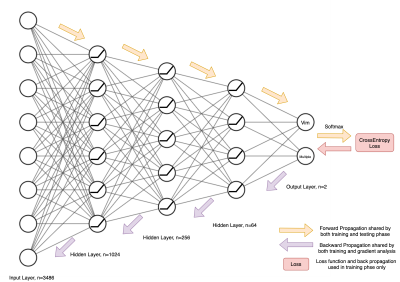
A convenience sample of 42 participants undergoing MRgFUS treatment was scanned using a 3-Tesla GE MRI scanner and grouped according to whether they responded to a single Vim ablation (N = 14) or needed multiple ablations (N = 28). Scans were acquired 1-7 days prior to treatment and consisted of a DWI acquisition (b-values: 0, 700, 1000, 2800 s/mm2, with 8, 25, 40 and 75 directions, respectively, FOV: 230 mm, 1.8 mm isotropic resolution), a diffusion image with reversed phase encoding direction to correct distortions, and a sagittal 3D T1-weighted image (TR: 8 ms, TE:3.2 ms, Flip Angle: 10° and resolution 1.2×0.5×0.5 mm). The image processing pipeline to generate structural connectomes is summarised in Figure 1 and was conducted using MRtrix34, 5. Connectomes were labelled based on whether lesioning was performed in the Vim only, or Vim plus additional regions. Due to the symmetric nature of connectomes, only the upper triangle of the corresponding adjacency matrices was used for training and inference. Connection strength values were then normalised between 0 and 1 and subjects were split for cross-validation. A neural network of 4 fully connected layers (Figure 2) was trained and tested separately on each fold using the cross-entropy loss function to optimise the network weights. After inferencing, an analysis was performed on the correctly predicted subjects to determine the most relevant regions and connections for the treatment outcome. For each prediction, the gradient of the correct outcome with respect to the input connection was tracked. The inputs were then multiplied by these gradients and the values were normalised to show the relevance of each connection. The weighted degree of each node was then calculated by averaging all the weighted inputs of a given region. This weighted degree was then also normalised to show the relevance of each region.Results
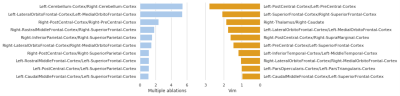
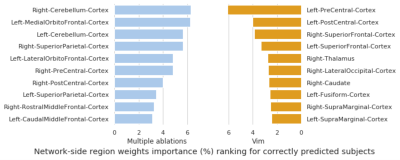
The trained network reached an overall prediction accuracy for all folds of 73.81%: subjects with Vim and multiple ablations were predicted with 71.43% and 75.00% accuracy, respectively. The confusion matrix of the overall performance is shown in Figure 3. The analysis of the network weights and brain connections and regions is shown in Figure 4 and Figure 5, respectively. The connection results revealed a relevant connection between the cerebellum cortex hemispheres and the left lateral orbitofrontal and medial orbitofrontal cortex in predicting participants that required multiple ablations (i.e., had other thalamic sites ablated after a Vim ablation to suppress tremor). Furthermore, the connection between the left postcentral cortex and the right superior frontal cortex was the most important connection for a patient to show tremor suppression after only a Vim ablation. Regarding the analysis of the brain regions, the right cerebellum cortex and the left medial orbitofrontal cortex had the highest contributions to predict the need for multiple ablation sites followed by the left cerebellum cortex and the right superior parietal cortex. The left precentral cortex was the most dominant factor to predict a good response to only a Vim ablation.Discussion
The key finding of this investigation was the accuracy with which a model naïve to the disease type could predict whether a subject underdoing MRgFUS would require lesioning outside of the Vim to achieve optimal tremor suppression. Moreover, the regions identified in the network analysis are all known to be important components of tremor networks in both ET and PD6. In particular, the cerebellum and pre-central gyrus are components of the cerebellar-thalamo-cortical network, which is thought to play a key role in tremor propogation3 and is disrupted by both Vim and PSA lesions. However, the exact relationship between connections to these regions and optimal targeting strategy will require further investigation.Conclusion
We proposed a neural network approach to predict the need for multiple MRgFUS ablations in tremor patients based on their structural connectivity. Whilst our analysis highlighted the importance of regions related to tremor disorders, further investigation is required to uncover the relevance of these findings for personalized treatment.Acknowledgements
The authors acknowledge the funding and expertise provided by GE Healthcare Australia and the assistance by the team at St Vincent's Hospital Sydney.References
[1] A. Jameel et al., “Double lesion MRgFUS treatment of essential tremor targeting the thalamus and posterior sub-thalamic area: preliminary study with two year follow-up,” Br. J. Neurosurg., vol. 0, no. 0, pp. 1–10, 2021, doi: 10.1080/02688697.2021.1958150.
[2] M. N. Gallay, D. Moser, and D. Jeanmonod, “Safety and accuracy of incisionless transcranial MR-guided focused ultrasound functional neurosurgery: Single-center experience with 253 targets in 180 treatments,” J. Neurosurg., vol. 130, no. 4, pp. 1234–1243, 2019, doi: 10.3171/2017.12.JNS172054.
[3] Y. Meng et al., “Technical Principles and Clinical Workflow of Transcranial MR-Guided Focused Ultrasound,” Stereotact. Funct. Neurosurg., vol. 99, no. 4, pp. 329–342, 2021, doi: 10.1159/000512111.
[4] Tournier J-D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;202:116137.
[5] Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology. 2012;22(1):53-66.
[6] R. S. Eisinger, S. Cernera, A. Gittis, A. Gunduz, and M. S. Okun, “A review of basal ganglia circuits and physiology: Application to deep brain stimulation,” Park. Relat. Disord., vol. 59, no. August 2018, pp. 9–20, 2019, doi: 10.1016/j.parkreldis.2019.01.009.
[7] Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014;103:411-426.
[8] Tournier JD, Calamante F, Connelly A. Improved probabilistic streamlines t ractography by 2nd order integration over fibre orientation distributions. Paper presented at: Proceedings of the international society for magnetic resonance in medicine2010.
[9] Smith RE, Tournier J-D, Calamante F, Connelly A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage. 2015;119:338-351.
[10] Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968-980.
Figures
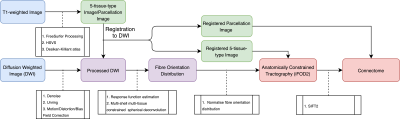
Generation pipeline of structural connectome. T1 images were pre-processed by FreeSurfer. Standard pre-processing was applied to DWI followed by estimation of the fibre orientation distribution in each voxel7. 10M streamlines8 followed by SIFT29 filtering were then performed. Desikan-Killiany atlas10 was used to create a structural connectome based on the streamlines and their SIFT2 weights.