1426
Time dependence at ultra-high diffusion weighting reveals fast compartmental exchange in rat cortex in vivo1Center of Functionally Integrative Neuroscience (CFIN) and MINDLab, Aarhus University, Aarhus, Denmark, 2Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark, 3Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal
Synopsis
The potential effect of compartmental water exchange and somas on the diffusion MRI signal in grey matter tissue are currently open questions with considerable implications for biophysical modelling of grey matter at (pre-)clinical diffusion time scales. Recent studies have begun to tackle these questions and found evidence that compartmental water exchange dominates the diffusion time dependence. Here, we extend the application of the Standard Model with exchange between the neurites and extracellular water (SMEX) to in vivo rat brain and again find evidence for exchange-dominated time dependence with very short neurite residence time on the order of 2 ms.
Introduction
Diffusion MRI (dMRI) can provide specificity towards important microstructural properties. Accordingly, considerable effort has been directed towards biophysical modeling of the dMRI signal. This is exemplified by the current consensus in white matter (WM) modeling in the form of the widely adopted standard model of diffusion1 (SM), which successfully treats WM as a collection of impermeable thin cylinders organized in fascicles throughout extracellular space2,3. A similar consensus is not established for grey matter (GM), where a better understanding of the effects of such features as compartmental water exchange and somas is currently needed4,5. Recent advances indicate that neurite exchange dominates the time dependence of the GM dMRI signal6,7 and that this time-dependence is well-described by the SM with exchange included between the neurites and extracellular water (SMEX7/NEXI6). Here, we present preliminary dMRI data extending the application of SMEX to in vivo rat brain and again find exchange to dominate the time dependence. The exchange time is estimated to be very fast, on the order of 2 ms.Methods
All experiments were preapproved by the competent institutional and national authorities and were carried out in accordance with European Directive 2010/63.Data acquisition: in vivo dMRI data for one Long Evans male rat was acquired on a 9.4T Bruker Biospec scanner equipped with a 86 mm volume transmit coil and a 4-element array reception cryocoil. The animal was induced with 5% isofluorane and maintained at 2% while monitoring breathing rate and temperature. The dMRI dataset was acquired using PGSE-EPI with TE=55.5ms, TR=2.5s, 4 averages, slice thickness=1mm, 19 slices, in-plane resolution=0.22x0.22mm2, matrix=100x82, per-slice triggering, and fat suppression. Diffusion parameters: four different diffusion times Δ = {11, 19, 27, 35} ms, constant gradient duration δ = 5.5ms, five diffusion weightings b = {1, 2.5, 5, 8, 11} ms/μm2 for Δ > 11 and b = {1, 2.5, 5, 6.5, 8} ms/μm2 for Δ = 11 ms. Each shell has 30 directions, and for each diffusion time there were 6 b=0 images.
Data analyses: We interpret the data using SMEX7 – the SM with exchange between the neurites and extracellular water with parameters: neurite exchange rate $$$r_n=1/\tau_n$$$, neurite and extracellular diffusivities $$$D_n$$$, $$$D_e$$$ (the extracellular diffusion is assumed isotropic), and signal fractions $$$f_n$$$, $$$f_e$$$. Somas are not explicitly accounted for8 but plausibly incorporated in the extracellular compartment due to sufficiently similar diffusion properties and exchange. A dot compartment is included. Parameters are estimated using least-squares fitting with starting point chosen by best initial fit amongst 107 random guesses. We analyze the powder-averaged signal from a large region of cortical grey matter (GM) delineated in three slices as specified in Fig. 1.
Results
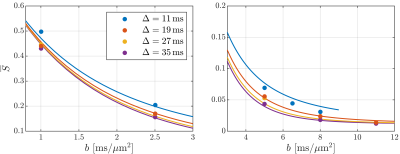
In Fig. 2, the cortical signal is seen to decrease as a function of diffusion time at fixed b-value though the time dependence is increasingly weak at larger pulse separation $$$\Delta$$$. It is also seen that SMEX captures the overall time-dependence well but has systematic deviation in the b-value decay. Fitted parameter values are given in Fig. 2 of which we highlight the neurite fraction $$$f_n$$$ = 73% and residence time $$$\tau_n$$$ = 2.3 ms.Fig. 3 shows maps of the neurite fraction and residence time throughout the considered region of cortex as estimated by voxel-wise application of SMEX. The parameters exhibit bilateral symmetry and stability within the cortex and with parameter values close to those from the average signal.
Discussion and conclusions
The decreasing signal with diffusion time at fixed b-value is a qualitative signature for compartmental exchange7 showing that exchange dominates the time dependence. In the case of exchange, the weaker time dependence at large $$$\Delta$$$ is consistent with expectations, because the exchanging compartments will become increasingly well-mixed and asymptotically appear as a single compartment.The fitted signal fractions $$$f_e$$$ = 26%, $$$f_n$$$ = 73%, $$$f_{dot}$$$ = 1.2% are consistent with histology4 and earlier reports7,9, but we note that the diffusivities $$$D_e$$$ = 3.0 μm2/ms, $$$D_n$$$ = 0.29 μm2/ms are surprisingly large and small respectively. The exchange time (∼2 ms) is on the same order but faster than recently found ex vivo in rat cortex7 (∼4 ms) as is possibly mediated by active transport in live tissue10. It is, on the other hand, smaller than recently reported also for live rat brain6 (∼20 ms). The very fast exchange found here has implications for GM modeling at clinical time scales (∼50 ms) where extracellular and neurite water may then be best described as well-mixed.
Limitations: This preliminary dataset included only a single animal and cannot capture inter-subject variability. Moreover, a potential confound is that the $$$b$$$ = 0 images were not acquired appropriately to accommodate correction for signal drift11. This has the potential to affect our conclusions because the data was acquired in order of pulse separation $$$\Delta$$$, which might then correlate with any drift. We are currently working on acquiring drift-corrected data with randomized ordering of $$$b$$$ and $$$\Delta$$$.
Acknowledgements
SJ and JO are supported by the Danish National Research Foundation (CFIN), and the Danish Ministry of Science, Innovation, and Education (MINDLab). Additionally, JO are supported by the VELUX Foundation (ARCADIA, grant no. 00015963). NS was supported in part by the European Research Council (ERC) (agreement No. 679058). AI is supported by ”la Caixa” Foundation (ID 100010434) and European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 847648, fellowship code CF/BQ/PI20/11760029. The authors acknowledge the vivarium of the Champalimaud Centre for the Unknown, a facility of CONGENTO which is a research infrastructure co-financed by Lisboa Regional Operational Programme (Lisboa 2020), under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund (ERDF) and Fundação para a Ciência e Tecnologia (Portugal), project LISBOA-01-0145-FEDER-022170.References
1. Novikov DS, Fieremans E, Jespersen SN, Kiselev VG. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. NMR Biomed. 2019;32(4)
2. Jespersen SN, Kroenke CD, Østergaard L, Ackerman JJH, Yablonskiy DA. Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage. 2007;34(4)
3. Kroenke CD, Ackerman JJH, Yablonskiy DA. On the nature of the NAA diffusion attenuated MR signal in the central nervous system. Magn Reson Med. 2004;52(5)
4. Jelescu IO, Palombo M, Bagnato F, Schilling KG. Challenges for biophysical modeling of microstructure. J Neurosci Methods. 2020;344:108861.
5. Novikov DS. The present and the future of microstructure MRI: From a paradigm shift to normal science. J Neurosci Methods. 2021;351:108947.
6. Jelescu IO, de Skowronski A, Palombo M, Novikov DS. Neurite Exchange Imaging (NEXI): A minimal model of diffusion in gray matter with inter-compartment water exchange. August 2021.
7. Olesen JL, Østergaard L, Shemesh N, Jespersen SN. Diffusion time dependence, power-law scaling, and exchange in gray matter. August 2021.
8. Palombo M, Ianuş A, Guerreri M, et al. SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. Neuroimage. 2020;215:116835.
9. Tax CMW, Szczepankiewicz F, Nilsson M, Jones DK. The dot-compartment revealed? Diffusion MRI with ultra-strong gradients and spherical tensor encoding in the living human brain. Neuroimage. 2020;210:116534.
10. MacAulay N. Molecular mechanisms of brain water transport. Nat Rev Neurosci 2021 226. 2021;22(6):326-344.
11. Vos SB, Tax CMW, Luijten PR, Ourselin S, Leemans A, Froeling M. The importance of correcting for signal drift in diffusion MRI. Magn Reson Med. 2017;77(1):285-299.
Figures