1425
Novel particle-based spatial stochastic Bloch simulation applied to LDH-mediated pyruvate conversion1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
In order to model signal mechanisms relevant to HP 13C MRI of lactate, we developed a novel particle-based MR model which extends the Brownian dynamics simulator Smoldyn. The model performs concurrent calculation of a forward solution of the Bloch equations for simulated particles using quaternion rotations. Reaction kinetics of LDH-mediated conversion of pyruvate to lactate were simulated and compared to a benchtop experiment (LDH activity assay). Modelling of particle compartmentalization and motion were also tested in simulated structures.
Introduction
Hyperpolarized carbon-13 MRI (HP 13C MRI) characterizes metabolism by monitoring changes in the MR signal associated with labelled metabolites as they undergo diffusion, transport, and metabolic reactions. Notably, 13C-lactate signal from the human brain is likely affected by compartmentalization of lactate dehydrogenase (LDH), the enzyme that catalyzes the interconversion between pyruvate and lactate. The design of MR experiments to understand the signal mechanisms arising from such metabolic processes necessitates specialized modelling to predict the effects of relevant parameters.The aim of this work was to develop a novel particle-based MR model and validate it by modelling the reaction kinetics of LDH-mediated conversion of pyruvate to lactate and comparing to a benchtop experiment. We implemented a particle-based Brownian dynamics simulator to represent biochemical reactions, spatial compartmentalization, and molecular motion more realistically than coarse-grained ODE-based or PDE-based methods,1,2 and incorporated a quaternion-rotation-based forward solution of the Bloch equations to model the MR signal associated with each simulated particle.
The model uses the Pulseq3 framework for pulse sequence input, and extends the spatial stochastic simulator Smoldyn4 with concurrent MR modelling for selected species within any biochemical simulation. We used this model to simulate particle diffusion within constrained spatial geometries as well as the kinetics of pyruvate-to-lactate conversion.
Methods
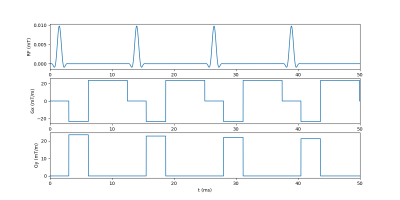
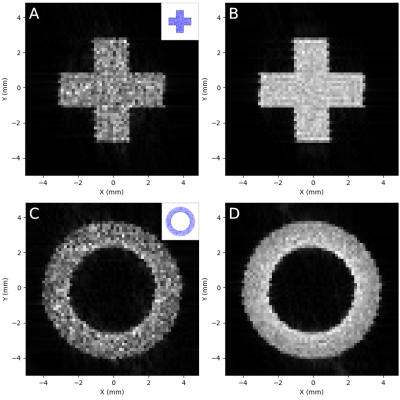
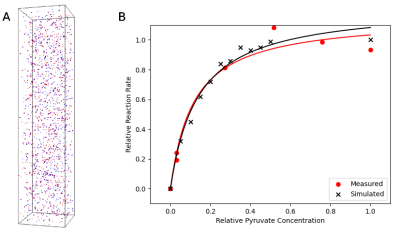
Conversion of pyruvate to lactate in the presence of LDH was measured by conducting a standard LDH activity assay. A modification of the protocol from Ref.5 was used. In brief, 0.25 mM reduced nicotinamide adenine dinucleotide (NADH) and 0.25 units/mL LDH were mixed in 2.25 mL sodium phosphate buffer (pH 7.4) along with increasing concentrations of pyruvate: 0.18, 0.22, 0.25, 0.28, and 0.32 mM. After addition of pyruvate, the solution was pipetted into a polystyrol cuvette and placed in a BioMate 3 spectrophotometer (Conquer Scientific). Absorbance at 340 nm was monitored at 1 s intervals for 5-10 minutes to measure loss of NADH. Following all measurements, the initial reaction rate from the linear part of each absorbance curve was plotted against the substrate concentration of pyruvate and the data was fit to a standard Michaelis-Menten enzyme kinetics model. Fitting was performed in Python via least-squares minimization. The fitted model was normalized using the calculated maximum reaction velocity and maximum substrate concentration for comparison to the simulated data (Figure 3).Simulations were configured in Smoldyn using the novel Smoldyn-MR (SMR) extension and conducted using two cores of a six-core CPU (AMD Ryzen 5 2600 Six-Core Processor, 3400 MHz). For the MR images (Figure 2), 10,000-100,000 randomly diffusing particles in constrained geometries were simulated for 0.8 seconds with a 10 μs time step. Total simulation run time for 100,000 particles was approximately 22 minutes. The pulse sequence (partially shown in Figure 1) was created using Pulseq’s MATLAB library: following application of a 90⁰ flip-down pulse, Cartesian k-space sampling was conducted to generate a 64x64 image. T1 was set at 0.4 s in all regions and a 1.0 s delay was added after each readout to allow longitudinal magnetization recovery. Images were reconstructed in Python using SciPy.
Spectroscopic measurements for kinetics were determined by simulating a 2.25 mL volume containing 100 NADH and LDH particles, with MR signal modelled for pyruvate particles with different starting concentrations (100-2,000 particles). Simulation and pulse sequence parameters were the same as above, but without gradients; the transverse magnetization following each flip-down pulse was used to monitor the decrease in pyruvate when converted to lactate. Fitting was performed as in the in vitro assay and results are shown in Figure 3.
Results & Discussion
Predicting and interpreting the results of metabolic imaging techniques such as HP 13C MRI requires insight into the effects of microstructure and reaction kinetics on MR signal mechanisms, and this model reproduces these effects. Reconstructed images from in silico experiments with structural constraints on particle motion, conducted with increasing numbers of particles, showed general detail of each mapped structure even at very low (<1,000) particle counts, and edge detail was clear in the >10,000 particle range (Figure 2).Simulated reaction kinetics also matched closely with measured values (Figure 3B), with a Fréchet distance between the two normalized kinetics curves of 0.05. This result is particularly important with respect to HP 13C MRI of the brain, as our group has previously identified a region-specific topography of 13C-lactate signal in vivo in the human brain.6 Accurate modelling of LDH-mediated interconversion between pyruvate and lactate is a crucial step toward general modelling of HP 13C MRI of brain lactate.
This model was designed as an extension to established software both to increase utility and to mitigate the computational costs of particle-based simulations; Smoldyn was chosen for its versatility and efficiency relative to comparable platforms.4,7 The addition of concurrent MR modelling eliminates the substantial time required to convert data between formats when particle trajectories are generated prior to MR simulation, as noted in previous diffusion MRI studies, where up to 100 hours per voxel was needed for conversion of Smoldyn outputs.8,9 Future integration of modules for GPU-based parallelization10,11 and multi-scale modelling7 should enable further speed improvements.
Acknowledgements
No acknowledgement found.References
1. Frazier, Z. & Alber, F. A Computational Approach to Increase Time Scales in Brownian Dynamics–Based Reaction-Diffusion Modeling. J. Comput. Biol. 19, 606–618 (2012).
2. Schöneberg, J., Ullrich, A. & Noé, F. Simulation tools for particle-based reaction-diffusion dynamics in continuous space. BMC Biophys. 7, 11 (2014).
3. Layton, K. J. et al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn. Reson. Med. 77, 1544–1552 (2017).
4. Andrews, S. S. Smoldyn: particle-based simulation with rule-based modeling, improved molecular interaction and a library interface. Bioinformatics 33, 710–717 (2017).
5. Tegge, G. Bergmeyer, H. U. (Editor-in-Chief): Methods of Enzymatic Analysis (Methoden der enzymatischen Analyse), 3rd Edition. Editors: J. Bergmeyer und Marianne Graßl. Volume III, Enzymes 1: Oxidoreductases, Transferases. Verlag Chemie, Weinheim – Deerfield Beach – Basel 1983. XXVI, 605 p., with 18 figs. and 43 tables. Hardcover-cloth DM 224,– (subscription price, when all 10 volumes are ordered) individual volume price DM 258,–. Starch - Stärke 37, 106–107 (1985).
6. Lee, C. Y. et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. NeuroImage 204, 116202 (2020).
7. Robinson, M., Andrews, S. S. & Erban, R. Multiscale reaction-diffusion simulations with Smoldyn. Bioinformatics 31, 2406–2408 (2015).
8. Bates, J., Teh, I., Kohl, P., Schneider, J. E. & Grau, V. Sensitivity Analysis of Diffusion Tensor MRI in Simulated Rat Myocardium. in Functional Imaging and Modeling of the Heart (eds. van Assen, H., Bovendeerd, P. & Delhaas, T.) 120–128 (Springer International Publishing, 2015). doi:10.1007/978-3-319-20309-6_14.
9. Bates, J. et al. Monte Carlo Simulations of Diffusion Weighted MRI in Myocardium: Validation and Sensitivity Analysis. IEEE Trans. Med. Imaging 36, 1316–1325 (2017).
10. Gladkov, D. V., Alberts, S., D’Souza, R. M. & Andrews, S. Accelerating the smoldyn spatial stochastic biochemical reaction network simulator using GPUs. in Proceedings of the 19th High Performance Computing Symposia 151–158 (Society for Computer Simulation International, 2011).
11. Dematte, L. Smoldyn on Graphics Processing Units: Massively Parallel Brownian Dynamics Simulations. IEEE/ACM Trans. Comput. Biol. Bioinform. 9, 655–667 (2012).
Figures