1421
Effect of brain tissue deformation on functional MRI signal variations assessed using biomechanical simulations
Mahsa Zoraghi1, Nico Scherf2,3, Carsten Jaeger1, Ingolf Sack4, Sebastian Hirsch5,6, Stefan Hetzer5,6, and Nikolaus Weiskopf1,7
1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Methods and Development Group Neural Data Science and Statistical Computing, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Institute for Medical Informatics and Biometry, Carl Gustav Carus Faculty of Medicine, TU Dresden, Dresden, Germany, 4Department of Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany, 5Berlin Center for Advanced Neuroimaging, Charité – Universitätsmedizin Berlin, Berlin, Germany, 6Berlin Center for Computational Neuroscience, Berlin, Germany, 7Faculty of Physics and Earth Sciences, Felix Bloch Institute for Solid State Physics, Leipzig University, Leipzig, Germany
1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Methods and Development Group Neural Data Science and Statistical Computing, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Institute for Medical Informatics and Biometry, Carl Gustav Carus Faculty of Medicine, TU Dresden, Dresden, Germany, 4Department of Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany, 5Berlin Center for Advanced Neuroimaging, Charité – Universitätsmedizin Berlin, Berlin, Germany, 6Berlin Center for Computational Neuroscience, Berlin, Germany, 7Faculty of Physics and Earth Sciences, Felix Bloch Institute for Solid State Physics, Leipzig University, Leipzig, Germany
Synopsis
Recent studies on the brain suggest a link between brain function and biomechanics of brain. In this study we develop a novel simulation framework to investigate blood flow-induced deformation caused by increased neural activity in brain tissue considering its mechanical properties. We further investigate its impact in simulated fMRI experiments. Our results demonstrate that the displacement in gyrus induced by local volume and stiffness change might not be directly resolved due to limited fMRI resolution, but can lead to artifacts when interpreting measurements at layer-level resolution. Our findings may help to systematically analyze potential resulting artefacts in high resolution fMRI.
Introduction
Viscoelastic properties of the human brain tissue vary slightly during hypercapnia-induced vasodilation1. The primary fMRI contrast mechanisms rely on neuronal-induced vasodilation response2. However, the mechanical effects of vasomotion in cortical structures are neither investigated nor considered in high resolution fMRI studies yet1,3. Even minor tissue displacements on the fMRI sub-voxel level can lead to erroneous relative fMRI signal changes of the same magnitude, indicating the importance of investigating these mechanical effects during vasomotion on the resulting fMRI signal which to our knowledge has not been studied systematically to date. Using biomechanical simulations, we investigated the influence of variations in brain tissue mechanical property, due to vasodilation on high resolution fMRI images (published recently4). First, we performed finite element simulation of brain tissue deformations in a gyrus of the human brain using ultra-high resolution histology data as input. Second, we simulated high-resolution fMRI datasets at multiple spatial resolutions and contrasts in order to investigate the effect of the estimated tissue deformations on the resulting fMRI signal.Methods
Anatomical ModelWe constructed a two-dimensional geometric model of a coronal slice of the right middle frontal gyrus obtained from ultra-high resolution histology data of 20 µm from the HumanBrainProject5. The six cortical layers in GM were manually traced. The input geometry consisting of surrounding GM, WM, and CSF was imported in COMSOL Multiphysics (V.5.5,COMSOL Multiphysics Göttingen) for finite element simulations. The biomechanics of CSF were not included in the simulations.
Biomechanical Simulations
The brain tissue in our study was modeled as neo-Hookean hyperelastic and assumed to be isotropic6 and nearly incompressible7. Literature values8,9 of brain tissue properties were used as input to the simulation model. Biomechanical simulations were performed to study the effect sizes of local volume increase, stiffness increase and the combination of both. In the first simulation scenario, the shear modulus of a segment within the first cortical layer as highlighted in red in Fig. 1 underwent an increase of up to 10% of its initial value in 20 equidistant steps of 0.5%. In the second scenario, the same segment underwent an increase in volume by uniformly extending its lateral dimensions in 0.5% steps. Finally, the increases in stiffness and volume were combined in a single simulation to study their combined effect.
Functional MRI Simulations
The gyrus geometry obtained from the biomechanical simulations was imported to MRiLab (http://mrilab.sourceforge.net/) to estimate the sensitivity of different high-resolution fMRI sequences to tissue displacement caused by vasodilation. The simulations included the three most commonly used fMRI variants: proton density contrast as used in arterial spin labeling (ASL), Vascular-Space-Occupancy (VASO) and blood-oxygen-level-dependent (BOLD) on a 7T MRI system and idealized RF coils and B0 homogeneity. The fMRI simulation parameters were taken from the literature10 and mapped to the mechanical gyrus model.
The magnitude MR images were constructed from the simulated k-space data at three 0.25, 0.5 and 1.0 mm isotropic resolutions for the simulated gyral geometries for volume increase.
From the corresponding MR images of the modeled gyrus in the resting and "activated" states, voxel-wise maps of the relative signal change caused by tissue displacement were calculated.
Results
Resulting tissue displacement for three simulation scenarios i.e. increase in stiffness, volume and the combined effects are shown in Fig. 2. One can see that the volume increase simulation resulted in a larger displacement compared to the stiffness change. The largest displacement was observed for the combined effect. For visualization purposes a scaling factor of 5 was chosen for the figure. Fig. 3 shows the cortical gyrus geometry in the resting state as used for fMRI simulations for idealized example VASO, BOLD and ASL protocols in different resolutions. The tissue displacement due to a 2% volume increase resulted in spurious fMRI signal changes. The results yielded the strongest signal change in methods based on images with high contrast between neighboring tissues and cortical layers. It should also be noted that the artifacts in signal changes increase with decreasing voxel size.Discussion and Conclusion
Our biomechanical simulation results showed remarkable tissue displacement resulting from blood flow-induced alterations in mechanical tissue properties. In our simulated fMRI measurements, these displacements resulted in significant artifacts. Our findings highlight the importance of considering and characterizing tissue displacement as a potential source of fMRI artifacts. Although our simulation study requires validation, it provides insights into potential biomechanical effects and how they may impact high resolution fMRI measurements.Acknowledgements
The research leading to these results has received funding from German Research Foundation (Exc 257 #276880906; #39052203) and the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905. NW has received funding from the European Union's Horizon 2020 research and innovation programme under the grant agreement No 681094 and the BMBF (01EW1711A & B) in the framework of ERA-NET NEURON.References
Figures
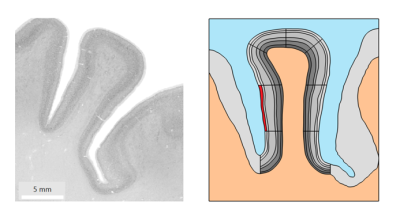
Figure 1. Histology image of the right middle frontal gyrus on the left taken from HumanBrainProject used as input geometry for biomechanical simulations. The imported geometry for simulations and the segment of interest for simulations are highlighted in red on the right.
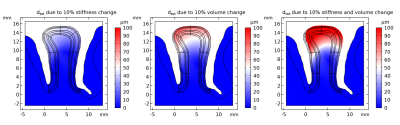
Figure 2. Tissue displacement resulting from a 10% increase of tissue stiffness, volume and their combined effect for the studied segment with the largest displacement in the combined simulation scenario. A scaling factor of 5 was chosen for visualization.
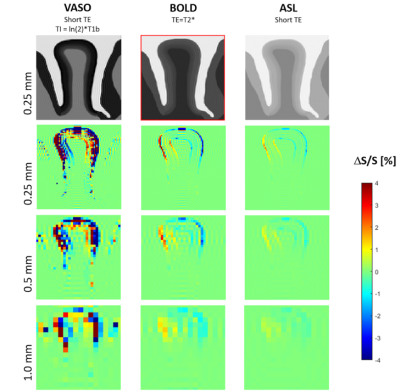
Figure 3. Simulated MR images of three different idealized fMRI VASO, BOLD and ASL protocols with their corresponding maps of signal change (due to tissue displacement resulting from a 2% volume increase of the studied segment) shown for three different spatial resolutions.
DOI: https://doi.org/10.58530/2022/1421