1407
Intravoxel Incoherent Motion (IVIM) to study synovitis in Osteoarthritis: a feasibility study1Radiology, Stanford University, Stanford, CA, United States
Synopsis
Synovial inflammation (synovitis) is present in the majority of people with Osteoarthritis(OA) and has been linked with pain, disease severity and progression of knee OA. However, synovitis is not widely studied due to challenges differentiating synovial inflammation from adjacent fluid on conventional MRI and contraindications and time, cost and complexity of gadolinium-based contrast-enhanced methods. In this work, we evaluate the feasibility of the intravoxel incoherent motion(IVIM) imaging model for characterization of synovitis activity. By characterizing the diffusion from synovial fluid effusions, we showed that the intravoxel signal from effusions can be removed allowing isolation of diffusion effects from the synovium.
Introduction
Osteoarthritis (OA) is a leading cause of disability that reduces productivity and quality of life at tremendous economic cost(1). Synovial inflammation (synovitis) is present in the majority of people with OA and has been linked with pain, disease severity and progression of knee OA. However, a lack of comprehensive, non-invasive methods to assess synovitis has limited our ability to study the role of inflammation in whole-joint disease as well as evaluate the efficacy of therapeutic interventions. Conventional MRI struggles to differentiate synovial inflammation from adjacent fluid (combined signal called effusion synovitis). Contrast-enhanced (CE) MRI, following intravenous injection of a gadolinium contrast-agent (GbCA), provides contrast to the highly vascularized synovium and is the current reference standard for imaging synovitis. Further, Dynamic Contrast Enhanced (DCE) methods can add quantitative measures that reflect local synovial inflammatory activity. However, added time, cost, complexity, and contraindications in patients with renal dysfunction, have limited CE-MRI in OA. Diffusion tensor imaging (DTI) offers a novel approach to assess the synovial lining hyperplasia and increased vascularization that occur in inflamed synovium and has shown good correlation to DCE Ktrans measures(2). However, due to the relatively low resolution of DTI measurements, it suffers from partial volume effects of inflamed synovium with effusion. The intravoxel incoherent motion (IVIM) imaging model can decompose the diffusion signal into a slow (perfusion) and fast (effusion) contribution(3). As the perfusion signal offers a quantitative measure of inflamed synovium, isolation of perfusion component in effusion synovitis would robustly characterize inflammatory activity in the synovium without a contrast agent. In this work, we evaluate the feasibility of IVIM MRI for characterization of synovitis activity and discrimination of perfusion fraction in synovitis present in OA.Methods
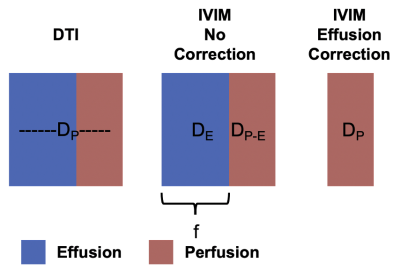
The knee of one subject with moderate to advanced OA was scanned on a 3T whole body scanner (GE SIGNA Premier) using a 16 channel flexible phased-array receive only coil. We acquired one IVIM and one DTI dataset. For the IVIM scan, we acquired one volume with b=0 s/mm2, together with diffusion encoded images along 4 directions (tetrahedral scheme). The b values used for each directions were 10, 20 , 50, 80, 100, 150, 200, 250, 300, 350 s/mm2. For the DTI scan, diffusion was encoded along 10 non-collinear directions with b = 400 s/mm2. Both scans were acquired with a 3-shots EPI readout (matrix size = (256,256,17); voxel size = 0.5 x 0.5 x 6 mm3; TR/TE = 3000/65 ms). Scan time was 5:08 for IVIM and 4:12 for DTI acquisitions. a Additionally, we acquired one DESS scan (matrix size = (256,256,50); Slice Thickness = 3.0 mm; TR/TE1/TE2 = 13.5/4.6/22.3 ms) for anatomical reference.The signal originating from a single voxel (Figure 1) as a function of b-value can be described by a bi-exponential model:
\[S(b)= S_{0}*fe^{-bD_{p}} + S_{0}*(1-f)e^{-bD_{e}}\:\:\: (Equation\:1)\]
Where Dp = perfusion and De = effusion and f = fraction of the perfusion component.
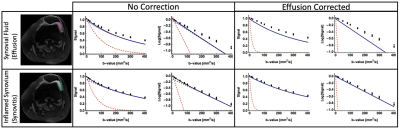
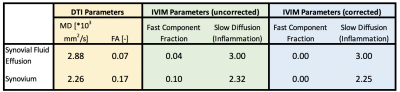
All diffusion images were denoised using a Principal Component Analysis algorithm(4,5) and non-rigidly registered to the reference DESS scan to correct for EPI-induced image distortions. For DTI, we fitted the data using a WLLS algorithm. The full IVIM model (Equation 1) was fit to the average signal per b-value using a NLS algorithm, with initialization parameters: (S0 = 1, f = 0.05, Dp = 0.003, De=0.01). The isotropic effusion component of the signal was calculated and subtracted from the signal of the IVIM dataset, which were subsequently fitted again to the same models (Figure 1). Regions of interest were drawn in the inflamed synovium and in synovial fluid (Figure 2) and used to extract diffusion parameters. All image analysis was performed using QMRITools for Mathematica (6).
Results
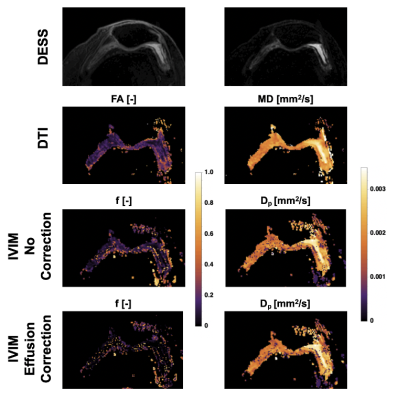
In the effusion, we observed a mono-exponential signal decay with high diffusion, clearly indicating free gaussian diffusion. On the other hand, we observed a bi-exponential decay in the synovium, originating from the contribution of effusion and perfusion (Figure 3). The DTI parameters had the worst contrast between the two tissues, while Dp provided high contrast. The correction successfully eliminated the contribution from the fast effusion component, as indicated by the mono-exponential fit and by the corrected f map. The diffusion parameters derived using the IVIM model suggest its ability to successfully decouple the two components.Discussion
We demonstrated the feasibility of IVIM imaging for characterizing synovial inflammation including removing confounding effects from partial-volumed synovial effusions. Previous work has shown the potential for characterizing synovial inflammation using DTI, including a strong correlation with DCE Ktrans measures, but relied on CE-MRI to differentiate synovium from synovial fluid. By characterizing the contribution from effusion signal in each voxel, the contribution from synovial fluid can be corrected, allowing an IVIM measure of diffusion solely due to microvascular flow in synovium. We will further refine our method by taking the different relaxation times of synovium and effusion into account.In this proof-of-concept study, we demonstrated that IVIM acquisition can be performed across the whole knee in roughly 5 minutes using a multishot EPI readout, supporting the clinical feasibility of this method. Further work is needed to evaluate this approach in a larger cohort as well as its sensitivity to varying synovial hypertrophy and inflammation.
Acknowledgements
We receive research support from GE Healthcare.References
[1] Ma, V. Y., Chan, L. & Carruthers, K. J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Archives of physical medicine and rehabilitation. 2014;95(5):986-995
[2] Sandford HJC, MacKay JW, Watkins LE, Gold GE, Kogan F, Mazzoli V. Gadolinium-free assessment of synovitis using diffusion tensor imaging. NMR Biomed. 2021 Sep 21:e4614.
[3] Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505.
[4] Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016 Nov 15;142:394-406.
[5] Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. 2016 Nov;76(5):1582-1593.
[6]Froeling, (2019). QMRTools: a Mathematica toolbox for quantitative MRI analysis.. Journal of Open Source Software, 4(38), 1204, https://doi.org/10.21105/joss.01204
Figures