1397
Improved 3D stack-of-spiral UTE pulmonary imaging at 0.55T using iterative concomitant field and motion corrected reconstruction (iCoMoCo)1Cardiovascular Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Pulmonary Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Siemens Medical Solutions USA Inc, Malvern,, PA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, United States
Synopsis
Lung MRI using a high-performance 0.55T system offers reduced susceptibility artifacts, but high resolution imaging is challenged by low proton density, reduced signal-to-noise ratio, and concomitant-fields. 3D spiral acquisitions can be used for improved efficiency and SNR, at the expense of concomitant field related blurring. We present a self-gated, ultra-short echo time, stack-of-spirals acquisition with an efficient inline iterative reconstruction (<15 min) that incorporates both concomitant-field and motion-correction to enable robust high-resolution lung imaging at 0.55T. Our optimized and efficient imaging method is applied to improve image quality in healthy volunteers, patients with lung nodules, and patients with lymphangioleiomyomatosis (LAM).
Introduction
We recently demonstrated a stack-of-spirals ultra-short echo time (UTE) method for high-resolution pulmonary imaging at 0.55T1. This method used 40% of the data in a stable respiratory phase, resulting in low acquisition efficiency. An iterative motion-corrected (iMoCo) reconstruction framework2 can significantly improve the acquisition efficiency of our technique to improve SNR or reduce scan duration. Concomitant fields can be a source of significant image blurring in large field of view spiral imaging at 0.55T and need special consideration1,3. In this work, we propose a new concomitant field and motion corrected reconstruction (iCoMoCo) for stack-of-spiral pulmonary imaging at 0.55T and present a fast reconstruction which can produce images at scan time. Our technique leverages robust self-gating and trajectory corrections to produce high quality images in both healthy volunteers and patients.Methods
Data Acquisition: A prototype free-breathing 3D T1-weighted UTE spoiled gradient-echo stack-of-spirals sequence was used for imaging on a 0.55T MRI system (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany)4. Additional readouts were acquired along the superior-inferior (SI) direction to estimate the respiratory signal with temporal resolution of <300ms. All scans used golden-angle increments of spiral interleaves with FOV=45x45x19.6cm3 (LAM: 45x45x26.4cm3), resolution=1.75x1.75x1.75mm3 (LAM: 1.4x1.4x6 mm3), readout-duration=5.4ms (LAM: 3ms), acquisition-time=9.5min, peak-gradient-amplitude=26mT/m (LAM: 33.9mT/m).Reconstruction: Respiratory waveforms were extracted from the SI-readouts using previously published methods1. Data was retrospectively sorted into 8 equal bins and image reconstruction was performed in two steps:
Step 1: Respiratory resolved images from each respiratory bin were reconstructed using constrained reconstruction with spatial and temporal total-variation (TV). These images were registered to an end-expiration reference image and the motion fields5 (Mk) were estimated for reconstruction.
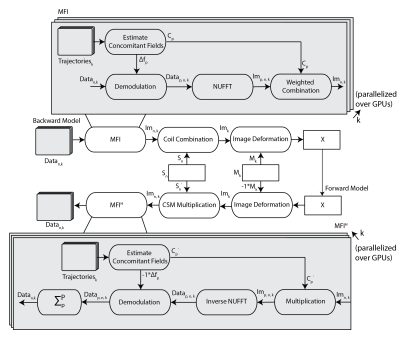
Step 2: The motion fields were used to perform a motion corrected reconstruction. Simultaneously, concomitant field correction was achieved using multi-frequency interpolation (MFI)6; weights and demodulation frequencies were calculated using previously published methods. Figure 1 shows the forward and backward model of the iCoMoCo reconstruction used to solve the following cost function,
$$argmin_X \sum_{n,k}^{N_c,K} ||{W_k \sum_p^P(D_pFC_p)S_nM_kX-d_{n,k}}||_2^2 + \lambda_s TV_s (X)$$
where X is the reconstructed image, dnk is motion resolved multi-channel data, Cp is MFI weights, Mk is estimated motion field, Sn is coil-sensitivity of the nth channel, F is the non-uniform Fourier transform, Wk is the density compensation weights for each motion state, Dp is the demodulation operator at a given frequency for MFI, and TVs is the spatial total variation term. Nc represents total number of channels, K is the total number of motion states, and P is the total number of demodulation frequencies.
Data were reconstructed inline using Gadgetron7 on a system equipped with Dual AMD EPYC processors, 1TB RAM, 4x Nvidia A100 GPUs. The reconstruction was parallelized over all GPUs to minimize reconstruction time. Density compensation weights were estimated using an iterative method8. Motion correction was performed within the Gadgetron framework5. Inaccuracies in spiral trajectories were corrected using gradient system impulse response functions9,10. For comparison images were also reconstructed using our concomitant-field corrected CG-SENSE reconstruction with 40% stable-binned data1. Apparent SNR (aSNR) was calculated as the ratio of mean signal in the lung parenchyma divided by the standard-deviation of a noise-only region.
Patient Imaging: In this IRB approved study, we demonstrate our method in four healthy volunteers, one patient with lymphangioleiomyomatosis (LAM), a rare cystic lung disease, and one patient with lung nodules. Images were acquired in either coronal or axial views. Data was retrospectively under-sampled to simulate scan-times of 2, 4.5, 7, and 9.5 min. aSNR was calculated for stable-binned CG-SENSE and iCoMoCo reconstruction using data from four healthy volunteers, for each scan-time.
Results
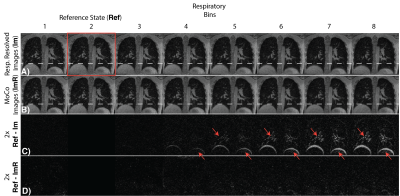
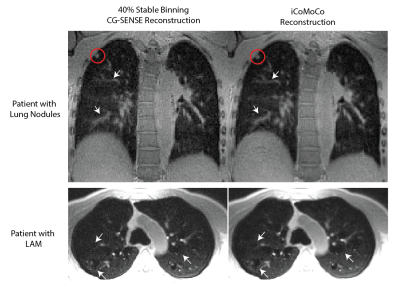
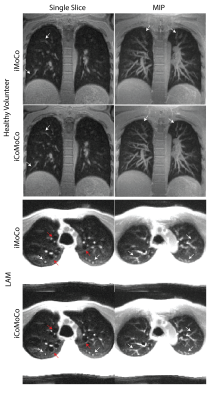
All reconstructions were completed in less than 15 min after the end of scan. Figure 2 shows an example of the respiratory resolved images before and after motion correction to demonstrate robustness of step 1 of the reconstruction, which is critical for iCoMoCo.Figure 3 qualitatively demonstrates the improvement in SNR with iCoMoCo reconstruction for data acquired in a patient with lung nodules and LAM. Figure 4 demonstrates the improvement in image quality with iCoMoCo reconstruction compared to iMoCo reconstruction for a HV and a patient with LAM. Significant improvement in vessel sharpness and cyst delineation highlight the importance of concomitant field correction at 0.55T.
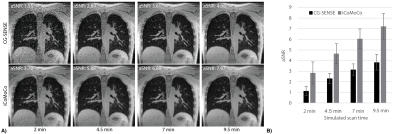
Figure 5 compares image quality between CG-SENSE and iCoMoCo for scan-times of 2-9.5 min. It shows that iCoMoCo images with scan-time of 4.5 min have comparable image quality to CG-SENSE images acquired in 9.5 min. Figure 5B shows aSNR was measured to be 2.83±1.04, 4.65±0.93, 6.06±0.92, and 7.22±1.20 for iCoMOCO and 1.14±0.42, 2.31±0.48, 3.15±0.53, and 3.84±0.74 for CG-SENSE reconstruction for simulated scan-times of 2, 4.5, 7, and 9.5 min.
Discussion and Conclusion
We have presented a fast inline iCoMoCo reconstruction for high-resolution 3D stack-of-spiral UTE pulmonary imaging on a 0.55T system. We demonstrate that a combination of robust respiratory binning, trajectory corrections, concomitant-field correction and motion correction are necessary for efficient and diagnostic-quality pulmonary imaging at lower field strengths. Using our optimized method, we were able to produce high-quality structural lung images during scan time in less than 15 min. iCoMoCo can improve pulmonary imaging at 0.55T by improving aSNR for a given scan time and by enabling shorter scan times for improved patient comfort.Acknowledgements
The authors thank Christine Mancini, Kendall O’Brien and Amanda Potersnak for their imaging expertise and Scott Baute, Margaret (Peg) Lowery, Jennifer Henry, Amelia Nargozian, Patricia Julien-Williams and Amanda Jones for assistance with patient recruitment. The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. The study was supported by the Intramural Research Program, NIH/NHLBI (Z01-HL006257).References
1. Javed A, Ramasawmy R, O’Brein K, et al. Self-gated 3D Stack-of-Spirals Ultra-Short Echo-Time Pulmonary imaging at 0.55T. Magn Reson Med. 2021. doi:DOI:10.1002/mrm.29079
2. Zhu X, Chan M, Lustig M, Johnson KM, Larson PEZ. Iterative motion-compensation reconstruction ultra-short TE (iMoCo UTE) for high-resolution free-breathing pulmonary MRI. Magn Reson Med. 2020;83(4):1208-1221. doi:10.1002/mrm.27998
3. King KF, Ganin A, Zhou XJ, Bernstein MA. Concomitant gradient field effects in spiral scans. Magn Reson Med. 1999;41(1):103-112. doi:10.1002/(SICI)1522-2594(199901)41:1
4. Guetter C, Xue H, Chefd’ Hotel C, Guehring J. Efficient symmetric and inverse-consistent deformable registration through interleaved optimization. Proc - Int Symp Biomed Imaging. 2011:590-593. doi:10.1109/ISBI.2011.5872476
5. Man LC, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn Reson Med. 1997;37(5):785-792. doi:10.1002/mrm.1910370523
6. Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med. 2013;69(6):1768-1776. doi:10.1002/mrm.24389
7. Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn Reson Med. 1999;41(1):179-186. doi:10.1002/(SICI)1522-2594(199901)41:1
8. Vannesjo SJ, Graedel NN, Kasper L, et al. Image reconstruction using a gradient impulse response model for trajectory prediction. Magn Reson Med. 2016;76(1):45-58. doi:10.1002/mrm.25841
9. Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med. 2016;75(6):2278-2285. doi:10.1002/mrm.25788
Figures