1393
White matter perivascular space enlargement in cerebral small vessel disease indicates cerebral amyloid angiopathy pattern changes1the second affiliated hospital of zhejiang university, school of medicine, hangzhou, China, 2radiology, the second affiliated hospital of zhejiang university, school of medicine, hangzhou, China
Synopsis
The enlargement of white matter perivascular space(PVS) is highly CAA related, however lack of studies investigated its role in stable pattern CSVD patients, we hypothesis that CSVD patients with white matter PVS enlargement indicates a CAA pattern changes.
Here we compared patients with and without white matter PVS enlargement from imaging manifestation, imaging mechanism and clinical cognitive ability. We foud that patients with PVS enlargenmet showed a pattern similar to previous symptomatic CAA, that is more lobar microbleed, more interstitial fluid retention and significantly lower global cognitive ability. Extra dignosis and treament should be carried out to this pattern CSVD.
introduction
Cerebral small vessel disease (CSVD) is highly prevalent and a major cause of stroke and dementia. The etiologies of CSVD are heterogeneous, and mainly include arteriosclerosis (ageing and hypertension-related) and cerebral amyloid angiopathy (CAA).The location of perivascular enlargement was recently suggested reflecting different etiologies. While basal ganglia perivascular space (PVS) enlargement was associated with arteriosclerosis, the PVS enlargement of white matter is thought to be a marker of cerebral amyloid deposition (CAA) type of CSVD or indicated amyloid deposition in cortical and leptomeningeal vessels. According to Boston criteria, the diagnosis of CAA is based on lobar cerebral haemorrhage patients, therefore most studies based on CSVD patients without haemorrhage only considered basal ganglia PVS enlargement and neglected white matter PVS enlargement. CAA pattern was different from the arteriosclerosis pattern. From the perspective of cognitive performance, widespread studies focused on symptomatic CAA found that it involved widespread cognitive areas, and was not only limited in executive function. From the view of the physiopathological mechanism, CAA is present with lobar microbleeds due to the amyloid deposition in cortical and leptomeningeal vessels. Furthermore, CAA as elimination failure angiopathies also demonstrated much more interstitial fluid retention. We hypothesis that white matter PVS enlargement as an indicator of amyloid deposition in cortical vessels might show a pattern similar to previously determined symptomatic CAA. Therefore, in this study, we compared CSVD patients with and without white matter PVS enlargement of cognitive impairment pattern, imaging manifestation, and the retention of interstitial fluid status using advanced imaging technology.
Methods
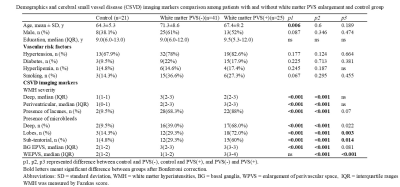
Twenty-five patients with white matter perivascular space (PVS) enlargement, 41 patients without white matter PVS enlargement, and 21 control participants were included. CSVD imaging markers were evaluated according to respective image standards. Cognitive ability including global cognitive, working memory, and processing speed were evaluated. Interstitial fluid retention evaluated by the free-water model was also carried out.Results
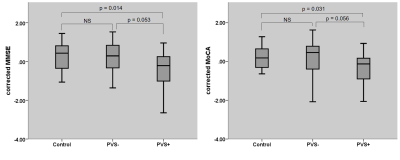
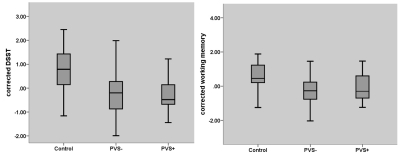
CSVD patients with white matter PVS enlargement showed more lobar and sub-tentorial microbleeds, than patients without. More posterior white matter hyperintensity distribution was demonstrated in CSVD patients with white matter PVS enlargement than patients without. Interstitial fluid retention was also significant higher in patients with white matter PVS enlargement. Cognitively, patients with PVS enlargement showed significant lower global cognitive ability, while patients without PVS enlargement showed relatively preserved global cognitive ability and significant lower executive ability.Conclusion
White matter enlarged perivascular spaces did have a great role in CSVD patients. It indicated a different pattern of CSVD, most probable with cerebral amyloid angiopathy, additional examination should be recommended to this kind of patient, therefore for better treatment.Acknowledgements
NoneReferences
Martinez-Ramirez S, van Rooden S, Charidimou A, et al. Perivascular Spaces Volume in Sporadic and Hereditary (Dutch-Type) Cerebral Amyloid Angiopathy. Stroke. 2018;49(8):1913-1919. doi:10.1161/STROKEAHA.118.021137
Shams S, Martola J, Charidimou A, et al. Topography and Determinants of Magnetic Resonance Imaging (MRI)-Visible Perivascular Spaces in a Large Memory Clinic Cohort. J Am Heart Assoc. 2017;6(9):e006279. Published 2017 Sep 22. doi:10.1161/JAHA.117.006279
Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88(12):1157-1164. doi:10.1212/WNL.0000000000003746
Figures
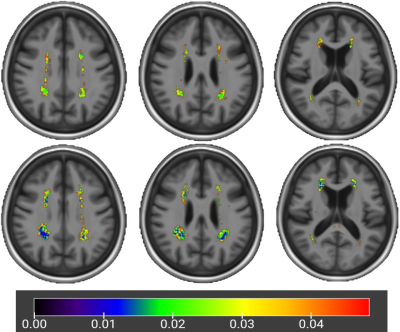
voxel-based white matter distribution difference from the control group
upper: significant p map of patients without white matter PVS enlargement
lower: significant p map of patients with white matter PVS enlargement