1392
In vivo, high-resolution Black Blood MRI at 7T1Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), Department of Radiology and Neuroradiology, University Medical Center Schleswig - Holstein, Kiel University, Kiel, Germany, Kiel, Germany, 2Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein, Kiel University, Kiel, Germany, Kiel, Germany
Synopsis
Vessel wall imaging (VWI) is a unique method to depict the vessel walls in vivo but was not applied in small animal models at 7T yet. Here, we developed a setup to emulate T1 contrast and flow to optimize Black Blood MRI in vitro. Using the results, we implemented a RARE sequence that allows in vivo Black Blood MRI with a high resolution of 0.156 x 0.156 mm² and successful flow suppression. Next, the model will be extended for VWI to measure the enhancement of Gadolinium contrast agent.
Introduction
Whereas techniques such as computed tomography angiography, MR angiography and digital subtraction angiography readily provide information about the vessel lumen1, MR vessel wall imaging (VWI) is unique in providing information about the vessel wall - in vivo and non-invasively. VWI is the only method allowing direct visualization of inflammation or damage of the vessel wall and, therefore, can resolve intracranial vasculopathies.²VWI is based on a sequence with strong flow suppression (black-blood MRI to obtain a dark vessel lumen) and T1 weighting to show Gadolinium (Gd) enhancement in the vessel walls.
While VWI is readily available for human MRI, it was not yet demonstrated for rodents. The challenges of translating VWI to mice and rats are smaller anatomy, a higher heart rate, and higher field strength.
Thus, the goal of this work was to implement VWI on a preclinical MRI scanner and test the method in vitro and in vivo. To this end, we developed a model for optimizing T1 contrast that allowed for a continuous flow variation. With these results, we obtained high-resolution black blood (BB) MRI in a healthy rat.
Methods
VWI model setup. To evaluate VWI in vitro, a setup for holding four 5 mm NMR tubes and one 6 mm diameter silicone tube was 3D printed (Form 3, formlabs) and filled with 1%-agarose gel (Figure 1a). The silicone tube was placed in the center and connected to a peristaltic flow pump (~5 cm/s, Masterflex, Computerized Drive, Cole Parmer). Four NMR tubes were filled with oil, water, Gd:NaCl 1:4000 and 1:8000 vol%.MRI. A 7T preclinical MRI and a 86 mm quadrature volume transmit-receive coil was used for imaging the in vitro model and abdomen, and a surface coil was added to image the brain (BioSpec 70/30, Bruker).
In vivo MRI. Healthy rats were anaesthetized by Ketamine and Medetomidine i.p. and placed on a heated animal bed. A pressure pad was used to monitor the breathing and trigger abdominal MRI.
Image analysis. Regions-of-interest (ROI) were used to quantify the flow suppression and flow artifacts (Paravision, Bruker). For flow suppression, the ROI was drawn at the position of the silicone tube (ROI 1, Fig. 1) to measure the signal intensity. For the flow artifacts, four ROIs were placed between the NMR tubes, two each in phase-encoding and readout direction, to calculate the standard deviation (std).
Results and Discussion
The newly developed model was readily set up in the MRI and allowed for investigating different flow rates, T1 contrasts and sequence parameters. Varying degrees of flow suppression and flow artifacts were observed in RARE MRI depending on the velocity (v) (Fig. 2), slice thickness (st) (Fig. 3) and echo time (TE) (Fig. 4).The flow suppression was found to decrease exponentially with increasing velocity. For v≈5cm/s, the signal of ROI 1 was up to ~eight times stronger than the noise level (poor suppression, TE=6 ms, st=0.8 mm). For velocities > 30 cm/s, the flow signal was as low as the noise level.
The flow suppression improved linearly from thicker to thinner slices, whereas the SNR decreased; for 0.2 mm, the signal in the flow ROI was equal to the noise level (v≈5 cm/s, TE=6 ms).
A similar trend was found for TE, where the noise level was approached for TE > 25 ms (v≈5 cm/s, st=0.8 mm). Both TE and slice thickness appear to be well suited to optimize flow suppression.
Prominent flow artifacts were observed in phase-encoding but not in readout direction (Fig. 1). Again, the artifacts were reduced for higher velocity, thinner slices and longer TE. The artifacts were eliminated for slice thicknesses of 0.3 mm – 0.2 mm at TE=6 ms, and for TE>25ms at a slice thickness of 0.8 mm, while T1 contrast was maintained (measured using the standard deviation in the ROIs).
These results suggested that 25ms TE and 0.8 mm slice thickness were well suited to perform BB MRI for a flow up to 5 cm/s with good T1 contrast, good SNR, acceptable scan time and no apparent artifacts.
In vivo, BB MRI of a rat brain and abdomen showed great flow suppression without any apparent artifacts (Fig. 5). In the brain, the flow in vessels with a diameter as small as 350 µm was well suppressed. A triggered acquisition was essential for imaging the abdomen; motion artifacts increased closer to the heart (not shown). Still, excellent flow suppression was achieved with high in-plane resolution and good SNR in the abdomen (Fig. 5a). Using relatively thick slices and long TE allowed short scan times while maintaining a high in-plane resolution, great flow suppression and reasonable scan time.
Conclusion and Outlook
Black Blood MRI in rats at 7T is feasible with a voxel size of 0.156 x 0.156 x 0.8 mm³.The presented setup was well suited to optimize BB MRI in vitro, and varying TE and slice thickness obtained an excellent flow suppression. Next, experiments will focus on further optimizing the resolution (e.g. isotropic, acceleration), testing different sequences, and demonstrating VWI using contrast agents and a model with vessel wall inflammation.
Acknowledgements
We acknowledge support by funding from the research training group “Materials for Brain” (GRK 2154) und MOIN CC. In addition, Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo. MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053).
References
[1] Mandell, D. M. et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 38, 218–229 (2017).
[2] Song, J. W. & Wasserman, B. A. Vessel wall MR imaging of intracranial atherosclerosis. Cardiovasc Diagn Ther 10, 982–993 (2020).
Figures
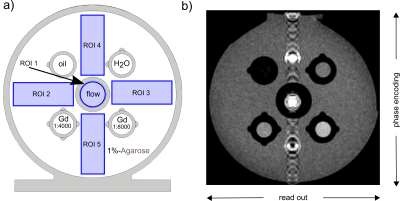
Figure 1. Sketch (a) and T1w RARE MRI (b) of the developed VWI model. The model was 3D printed to allow exchanging four NMR tubes and one flow tube in the center. Note the indicators printed to facilitate identification of the tubes and the five ROIs (blue) for quantification of the black-blood effect (ROI 1) and reduction of flow artifacts (ROI 2-5). In the MRI image (b), flow artifacts were visible in the phase-encoding direction.
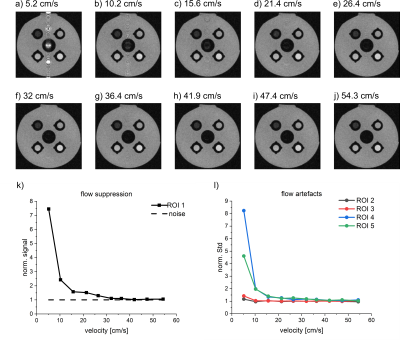
Figure 2. T1w RARE MRI of the VWI model for different flow velocities (a-j), quantification of flow suppression (k) and flow artifacts (l). The flow suppression is increased for higher velocities. A decreasing norm. signal of ROI 1 indicates an increasing flow suppression. A good flow suppression is achieved at st=0.2 mm, as the signal of ROI 1 equals the noise level (dashed line). Flow artifacts were only visible for smaller velocities. All MRI parameters were kept constant at TE=6 ms, echo train length=2, TR=750 ms, voxel size=0.35x0.35x0.8 mm³, FOV=45x45 mm².
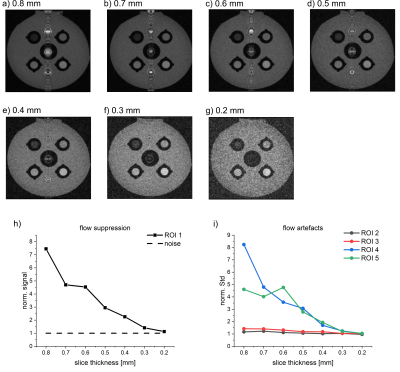
Figure 3. T1w RARE MRI of the VWI model for different slice thicknesses (a-g), quantification of flow suppression (h) and flow artifacts (i). The flow suppression was increased for thicker slices. A decreasing norm. signal of ROI 1 indicates an increasing flow suppression. A good flow suppression is achieved at st=0.2 mm, as the signal of ROI 1 equals the noise level (dashed line). Flow artifacts and SNR were decreased for thinner slices. All MRI parameters were kept constant at TE=6 ms, echo train length=2, TR=750 ms, pixel size=0.35x0.35 mm², FOV=45x45 mm², v≈5 cm/s.
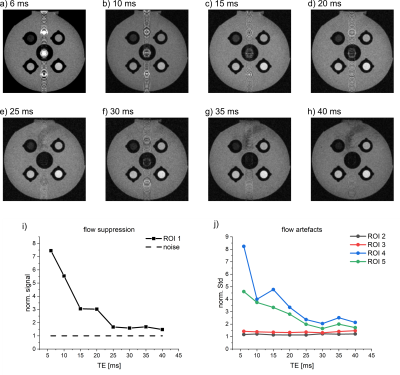
Figure 4. T1w RARE MRI of the VWI model for different echo times (a-h), quantification of flow suppression (i) and flow artifacts (j). The flow suppression was increased for longer TE. A decreasing norm. signal of ROI 1 indicates an increasing flow suppression. A good flow suppression is achieved at TE=25 ms, as the signal of ROI 1 approaches the noise level (dashed line). Flow artifacts were decreased for longer TE. All MRI parameters were kept constant at echo train length=2, TR=750 ms, voxel size=0.35x0.35x0.8 mm³, FOV=45x45 mm², v≈5 cm/s.
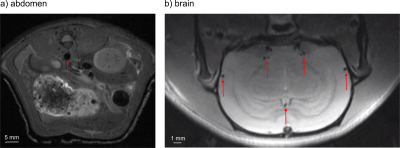
Figure 5. T1w in vivo Black Blood MRI at 7T in the rat abdomen (a) and brain (b). Note the different scales of the images. In both cases, no flow artifacts were apparent. All visible vessels (red arrows) show complete flow suppression.
For the abdomen the MRI parameters were TE=18 ms, echo train length=12, TR=750 ms, FOV=60x60 mm², voxel size=0.234x0.234x0.8 mm³, scan time=2:06 and for the brain TE=12 ms, echo train length=8, TR=750 ms, FOV=40x20 mm², voxel size=0.156x0.156x0.8 mm³, scan time=01:36.