1390
Quantification and visualization of aneurysm treatment outcome with signal enhancement on Black Blood MRI.1Department of Radiology and Neuroradiology, Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, 2Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel, Germany, 3Research Campus STIMULATE, University of Magdeburg, Magderburg, Germany
Synopsis
Intracranial aneurysm is a life-threatening disease that can be treated with flow-modulating devices (FMDs) by reducing aneurysm blood flow. Aneurysms might rupture after treatment, thus biomarker of successful treatment is needed. Flow MRI can detect flow reduction, however, it is impaired by metal artifacts from FMDs. Black-blood (BB) MRI is less sensitive to metal artifacts and often results in poor slow-flow suppression. We hypothesize that BB contrast changes after aneurysm treatment. Here, after placing FMDs in 3D-printed aneurysm models, an enhanced signal was found on BB MRI, associated with reduced aneurysmal flow after flow modulation and thus possible treatment success.
Introduction:
Intracranial aneurysm is a life-threatening disease that can be treated with flow-modulating devices (FMDs) by reducing the aneurysm blood flow. Aneurysms might rupture after treatment; thus a biomarker of successful treatment is needed.Flow MRI allows quantification of aneurysmal flow. The flow reduction was detected after aneurysm treatment1 and associated with successful treatment outcome2. However, flow MRI is impaired by metal artifacts originated from FMDs.
Black-blood (BB) MRI is less sensitive to metal artifacts and often results in poor slow-flow suppression. Earlier, a high BB MRI signal in the aneurysm lumen was associated with insufficient flow suppression and with slow-flowing blood3. We hypothesize that BB contrast changes after aneurysm treatment, and therefore, might serve as a biomarker of treatment outcome.
Thus, we aim to investigate whether results obtained from BB and flow MRI after aneurysm treatment correlate and could be used to determine aneurysm treatment success (Fig. 1). To this end, we assessed flow velocities and BB MRI signal before and after placing FMDs in vitro using 3D printed aneurysm models.
Methods:
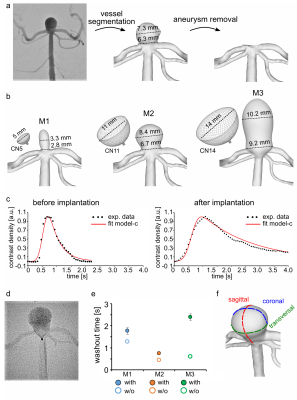
Aneurysm models (M1-3) were constructed in house4 from patient 3D rotational angiographic data (Fig. 2a). The vascular lumen was segmented, then the patient aneurysm was removed, and three artificial aneurysms were added (Fig. 2b). The models were 3D printed, submerged in an agarose gel, and connected to a pulsatile pump with a mean flow of 150ml/min.Models were treated by implanting three FMDs with a maximum diameter of 5, 11, and 14mm (Contour Neurovascular System, CN). The washout time of the angiographic contrast agent was quantified from time-density curves (Fig. 2c-e).
MRI measurements were performed on 3T MR system with a 32-channel head coil (Ingenia CX, Philips Healthcare). T1-weighted (T1w) BB 3D variable refocusing flip angle turbo spin echo sequence (TE/TR: 28/700 msec; FOV: 200×250×160mm3, voxel size: (0.65mm)3, echo train length: 55) was applied. Time-resolved phase-contrast (4D flow) MRI was acquired with 3D velocity encoding T1w spoiled fast gradient echo sequence with Cartesian sampling (TE/TR: 5/8.5msec; FOV: 100×110×40mm3; voxel size: (0.75mm)3; max. velocity-encoding: 75cm/s, 20 cardiac phases).
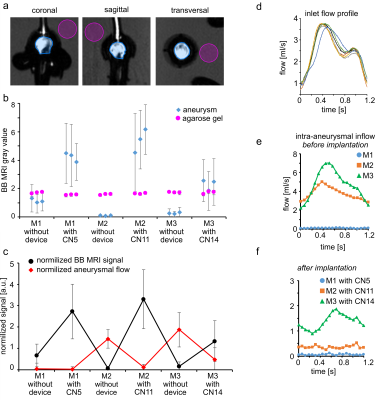
To quantify BB MRI signal, the aneurysm lumen was segmented in three perpendicular planes (Fig. 2f) and normalized by the signal of agarose gel. The aneurysmal flow was measured through a midplane across the aneurysm and normalized to the flow measured by an ultrasound sensor at the model inlet.
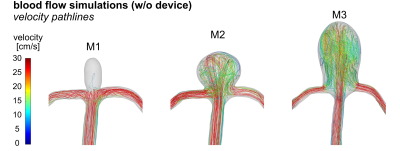
Blood flow simulations were done using computational fluid dynamics (CFD). Virtual vessels were based on 3D-printed aneurysm models. Inlet/outlet boundary conditions were set according to measured flow and pressure.
Results:
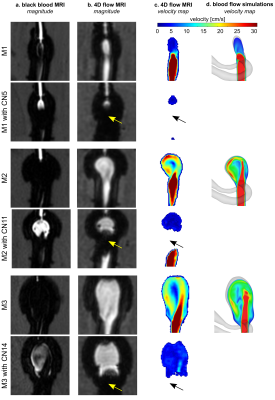
On CFD without FMDs, intra-aneurysmal flow in M1 was less than in M2-3 (Fig. 3), probably due to the smaller size of the aneurysm neck. On 4D flow MRI without FMDs, flow in M1 was less as well. On 4D flow MRI after placing FMDs, strong metal artifacts were present at the neck of the aneurysm, (Fig. 4b-c). Flow at the model inlet was similar before and after implantation, while the aneurysmal flow was reduced after implanting FMD (Fig. 5d-f). Flow reduction was also observed on DSA directly after implantation (Fig. 2c-e).On BB MRI a hyperintense signal was observed in the aneurysm lumen after endovascular treatment (Fig. 4a). BB MRI signal was similar among evaluation planes (Fig. 5a-b). The BB MRI signal in the aneurysm ROI was increased after the implantation of FMDs, while signal intensities in the agarose gel did not change.
The contrast of BB MRI and flow MRI is opposite: bright signal on BB MRI if the flow is slow, while the bright signal on 4D flow MRI if the flow is fast. Overall, placing of FMDs resulted in reduced flow and increased BB MRI signal for all models (Fig. 5c).
Discussion:
Increased BB MRI signal was found in aneurysm post-treatment, probably a consequence of reduced aneurysmal flow and poor slow-flow suppression. Flow reduction after FMDs placement was confirmed with 4D flow MRI and DSA. Previously, we have shown that BB MRI might fail to suppress slow flow in untreated aneurysms3. The current results demonstrate that this happens after treatment too.The high sensitivity of BB MRI to slow-flow might make it a valuable tool to assess flow reduction after aneurysm treatment. Note, that aneurysmal flow is commonly assessed with 4D flow MRI2, which provides comprehensive 3D velocity data. However, spin echo-based BB MRI is less sensitive to metal artifacts compared with gradient-based 4D flow MRI. Thus, BB MRI might be superior for flow analysis in the presence of metal implants. Previously, aneurysm healing was visualized with BB MRI after coiling, even though coils were placed into aneurysm cavity5.
Next, BB MRI signal needs to be assessed as a function of flow in a straight tube. To conclude on the clinical relevance of BB MRI for aneurysm treatment assessment, in vivo studies need to be conducted. Still, this study suggests that BB MRI might be used to visualize reduced flow, and consequently assess aneurysm treatment success.
Conclusion:
BB MRI can visualize reduced aneurysmal flow after flow modulation in vitro. It is less sensitive to metal artifacts than gradient-based methods. Thus, it might provide a clinically applicable non-invasive marker for successful aneurysm treatment.Acknowledgements
We acknowledge support by the RTG "Materials4Brain" (GRK2154; P2), Forschungscampus STIMULATE (13GW0473A), and the German Research Foundation (BE 6230/6-1).References
1. Pereira, V. M. et al. Assessment of intra-aneurysmal flow modification after flow diverter stent placement with four-dimensional flow MRI: a feasibility study. J. NeuroInterventional Surg. (2015) doi: 10.1136/neurintsurg-2014-011348
2. Brina, O. et al. How Flow Reduction Influences the Intracranial Aneurysm Occlusion: A Prospective 4D Phase-Contrast MRI Study. Am. J. Neuroradiol. (2019) doi:10.3174/ajnr.A6312
3. Pravdivtseva, M. S. et al. Pseudo-Enhancement in Intracranial Aneurysms on Black-Blood MRI: Effects of Flow Rate, Spatial Resolution, and Additional Flow Suppression. J. Magn. Reson. Imaging. (2021) doi: 10.1002/jmri.27587
4. Pravdivtseva, M. S. et al. 3D-printed, patient-specific intracranial aneurysm models: from clinical data to flow experiments with endovascular devices. Med. Phys. (2021) doi: 10.1002/mp.14714
5. Larsen, N. et al. Visualization of Aneurysm Healing. Clin. Neuroradiol. (2020) doi: 10.1007/s00062-019-00854-5
Figures