1387
Image quality of high-resolution three-dimensional neck MRI using CAIPIRINHA-VIBE and GRASP-VIBE: an intra-individual comparative study1Radiology, Seoul St. Mary's Hospital, Seoul, Korea, Republic of
Synopsis
The image qualities of two high-resolution MRI sequences of the neck—CAIPIRINHA-VIBE and GRASP-VIBE—were compared. 173 patients clinically indicated for neck MRI were scanned using both sequences with isotropic (<1 mm) in-plane resolution. The image quality was qualitatively assessed by two radiologists. Quantitative assessments (i.e., non-uniformity, contrast-to-noise ratio and signal-to-noise ratio) were performed in all patients, a phantom and a healthy volunteer. GRASP-VIBE outperformed CAIPIRINHA-VIBE in all qualitative assessments except for the fat suppression degree. Quantitative assessments were significantly superior in GRASP-VIBE than in CAIPIRINHA-VIBE. Therefore, GRASP-VIBE may be a better alternative to CAIPIRINHA-VIBE for head and neck MRI.
Introduction
Head and neck is one of the most challenging regions for MRI; due to abundant physiologic motions in the neck, fast acquisition techniques or special motion-insensitive protocols are needed to avoid severe image degradation.1,2 Also, high signal-to-noise ratio (SNR) is another crucial factor for sufficient image quality in fat-suppressed high-resolution neck MRI.3Recently, a novel MRI technique known as Golden-angle Radial Sparse Parallel imaging (GRASP) has been introduced for rapid free-breathing MR acquisitions.4,5 GRASP allows synergistic combination of compressed sensing, parallel imaging, and a gold-angle radial sampling scheme, providing iterative reconstruction for motion-insensitive images with variable temporal resolutions under free-breathing situation. Suggested shortcomings of GRASP are long post-processing time and streak artifacts especially seen in radial sequences.6
The purpose of current study was to qualitatively and quantitatively compare the image qualities of two high-resolution 3D MRI sequences of the neck—Controlled Aliasing in Parallel Imaging Results in Higher Acceleration-volumetric interpolated breath-hold examination (CAIPIRINHA-VIBE) and GRASP-VIBE.
Methods
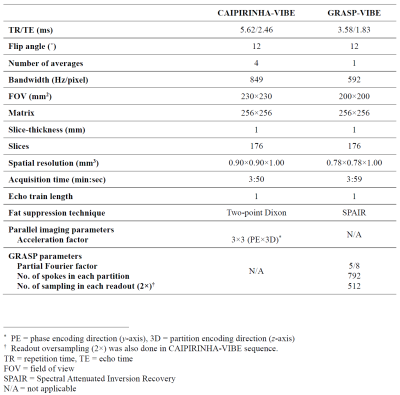
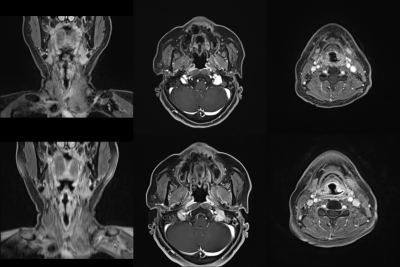
173 patients indicated for contrast-enhanced neck MRI were scanned using 3T scanners (Siemens Magnetom Vida). All patients were scanned using both sequences with nearly isotropic 3D acquisitions (<1 mm in-plane resolution) and analogous acquisition times (approximately 4 minutes). The GRASP-VIBE sequence was a prototype WIP (working in progress) sequence designed by Siemens Healthcare. The acquisition parameters of CAIPIRINHA-VIBE and GRASP-VIBE are summarized in Fig. 1.Patients’ MRI were independently rated by two radiologists using 5-grade Likert scale for overall image quality, overall artifact level, mucosal conspicuity, and fat suppression degree at nasopharyngeal, oropharyngeal and hypopharyngeal levels. Interobserver agreement was calculated using Cohen’s kappa. The quality ratings of both sequences were compared using Mann-Whitney U test.
Non-uniformity (NU) and contrast-to-noise ratio (CNR) were measured in all patients. Separate MRI scans were performed twice for each sequence in a phantom and a healthy volunteer without contrast injection to calculate SNR. Each value was calculated using following formulae:
$$NU={SD_{WM} \over SI_{WM}} ×100$$
$$CNR={SI_{WM}-SI_m \over SD_{air}}$$
$$SNR={mean(SI_1+SI_2)|_{ROI1} \over \sqrt{2}·SD(SI_1-SI_2)|_{ROI2}}$$
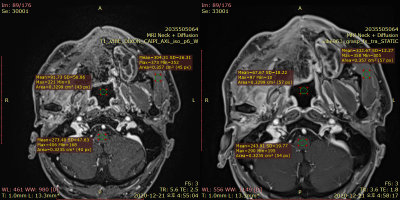
Here, WM refers to the white matter of the measured level, which is medulla oblongata at nasopharyngeal and oropharyngeal levels, and spinal cord at hypopharyngeal level. SIm indicates signal intensity (SI) of the muscle as a reference tissue, and SDair indicates standard deviation (SD) of the region of interest placed in the airway. SNR was calculated using the difference method with dual acquisition, with SI referring to the average of two separate scans and SD measured on the subtraction image.7–9 An example of NU and SNR measurement in the healthy volunteer is demonstrated on Fig. 2.
Results
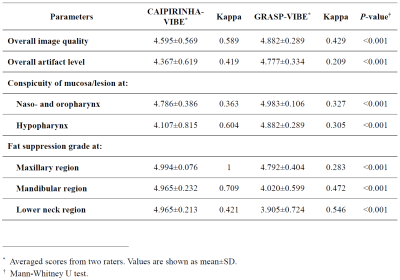
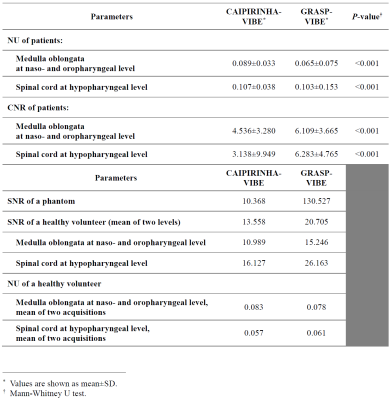
The quality ratings of patients’ MRI are summarized in Fig. 3. The scores of overall image quality, overall artifact level, conspicuity of the nasopharyngeal and hypopharyngeal mucosa were all significantly higher in GRASP-VIBE than in CAIPIRINHA-VIBE (all P-values < 0.001). Moderate interobserver agreement was observed in hypopharyngeal mucosal conspicuity of CAIPIRINHA-VIBE (κ = 0.60), overall image quality of CAIPIRINHA-VIBE (κ = 0.59), and overall image quality of GRASP-VIBE (κ = 0.43). The degree of fat suppression was weaker especially in the lower neck regions in GRASP-VIBE (3.90±0.72) than in CAIPIRINHA-VIBE (4.97±0.21) (P < 0.001).The results of quantitative assessment are shown in Fig. 4. The CNR at hypopharyngeal level was significantly higher in GRASP-VIBE (6.28±4.77) than in CAIPIRINHA-VIBE (3.14±9.95) (P < 0.001). On the phantom study, the SNR of GRASP-VIBE was 12 times greater than that of CAIPIRINHA-VIBE. In vivo SNR of the volunteer MRI was 13.6 in CAIPIRINHA-VIBE and 20.7 in GRASP-VIBE.
Discussion
GRASP-VIBE demonstrated significantly superior image quality for mucosal assessment with fair to moderate agreement between the two reviewers, whereas CAIPIRINHA-VIBE showed more uniform fat suppression. This result is consistent with the previous study, which reported better overall image quality and edge sharpness in GRASP.10 Also in line with most previous studies on free-breathing MRI, GRASP-VIBE showed higher scores in overall artifact level than CAIPIRINHA-VIBE (Fig. 5).10–14 Since GRASP and Dixon both require image post-processing, simultaneous use of both techniques required excessive post-processing time for routine clinical practice. Therefore, SPAIR (spectral adiabatic inversion recovery) was used in GRASP-VIBE as an alternative to the ideal Dixon fat suppression technique. This resulted in weaker fat suppression in GRASP-VIBE, compared to CAIPIRINHA-VIBE which used Dixon technique, as demonstrated in previous studies.15,16The quantitative assessments—overall SNR, CNR and NU at hypopharyngeal level— were better in GRASP-VIBE. The large difference in SNR between the two sequences might be due to high acceleration factor of CAIPIRINHA-VIBE, which was implemented to achieve motion-robustness. In vivo SNR of the healthy volunteer scan was also 1.5 times higher in GRASP-VIBE, which is concordant to the findings of previous studies.10,11,14 SI and SD were measured in the deepest structures possible to overcome the inhomogeneous noise distribution caused by parallel imaging, which would properly reflect “practically perceptible” noise for clinical interpretation. Streak artifacts at the hypopharyngeal level may have affected NU value on GRASP-VIBE images, considering the two sequences showed less noticeable distinction of NU at the hypopharyngeal level than at the nasopharyngeal level.
Conclusions
Both sequences rendered excellent images for head and neck MRI. GRASP-VIBE provided better image quality, mucosal conspicuities, SNR, and CNR whereas CAIPIRINHA-VIBE provided better fat suppression in lower neck regions.Acknowledgements
The authors greatly appreciate the technical support provided by a Siemens Healthineers MR physicist, Hyun-Soo Lee.References
1. Ruytenberg T, Verbist BM, Vonk-Van Oosten J, et al. Improvements in high resolution laryngeal magnetic resonance imaging for preoperative transoral laser microsurgery and radiotherapy considerations in early lesions. Front. Oncol. 2018;8(JUN):216.
2. Maroldi R, Ravanelli M, Farina D. Magnetic resonance for laryngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2014;22(2):131–139. Available at: https://journals.lww.com/co-otolaryngology/Fulltext/2014/04000/Magnetic_resonance_for_laryngeal_cancer.10.aspx. Accessed October 26, 2021.
3. Junn JC, Soderlund KA, Glastonbury CM. Imaging of Head and Neck Cancer With CT, MRI, and US. Semin. Nucl. Med. 2021;51(1):3–12.
4. Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest. Radiol. 2013;48(1):10–16.
5. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn. Reson. Med. 2014;72(3):707–717.
6. Block KT, Feng L, Grimm R, et al. GRASP : Tackling the Challenges of Abdominopelvic DCE-MRI. MAGNETOM Flash. 2014;5:16–22. Available at: www.siemens.com/magnetom-world. Accessed October 26, 2021.
7. Dietrich O, Raya JG, Reeder SB, et al. Measurement of signal-to-noise ratios in MR images: Influence of multichannel coils, parallel imaging, and reconstruction filters. J. Magn. Reson. Imaging. 2007;26(2):375–385. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/jmri.20969. Accessed July 23, 2021.
8. Reeder SB, Wintersperger BJ, Dietrich O, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: Application with cardiac imaging and a 32-channel cardiac coil. Magn. Reson. Med. 2005;54(3):748–754.
9. Fruehwald-Pallamar J, Szomolanyi P, Fakhrai N, et al. Parallel imaging of the cervical spine at 3T: Optimized trade-off between speed and image quality. Am. J. Neuroradiol. 2012;33(10):1867–1874. Available at: http://dx.doi.org/10.3174/ajnr.A3101. Accessed July 23, 2021.
10. Tomppert A, Wuest W, Wiesmueller M, et al. Achieving high spatial and temporal resolution with perfusion MRI in the head and neck region using golden-angle radial sampling. Eur. Radiol. 2020.
11. Seo N, Park SJ, Kim B, et al. Feasibility of free-breathing dynamic contrast-enhanced MRI of the abdomen: A comparison between CAIPIRINHAVIBE, Radial-VIBE with KWIC reconstruction and conventional VIBE. Br. J. Radiol. 2016;89(1066):1–7.
12. Shin HJ, Kim MJ, Lee MJ, et al. Comparison of image quality between conventional VIBE and radial VIBE in free-breathing paediatric abdominal MRI. Clin. Radiol. 2016;71(10):1044–1049. Available at: http://dx.doi.org/10.1016/j.crad.2016.03.018.
13. Chandarana H, Block TK, Rosenkrantz AB, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: A viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest. Radiol. 2011;46(10):648–653. Available at: https://journals.lww.com/investigativeradiology/Fulltext/2011/10000/Free_Breathing_Radial_3D_Fat_Suppressed.7.aspx. Accessed November 3, 2021.
14. Hur S-J, Choi Y, Yoon J, et al. Intraindividual Comparison between the Contrast-Enhanced Golden-Angle Radial Sparse Parallel Sequence and the Conventional Fat-Suppressed Contrast-Enhanced T1-Weighted Spin-Echo Sequence for Head and Neck MRI. Am. J. Neuroradiol. 2021. Available at: http://dx.doi.org/10.3174/ajnr.A7285. Accessed October 20, 2021.
15. Gaddikeri S, Mossa-Basha M, Andre JB, et al. Optimal Fat Suppression in Head and Neck MRI: Comparison of Multipoint Dixon with 2 Different Fat-Suppression Techniques, Spectral Presaturation and Inversion Recovery, and STIR. Am. J. Neuroradiol. 2018;39(2):362–368.
16. Yao G, Liang Z, Yang Y, et al. Comparison of fat suppression effects between Dixon and SPAIR techniques in the neck MRI. Chinese J. Radiol. 2020;54(7):707–712.
Figures