1379
Meta-Analysis of the Normal Diffusion Tensor Imaging Values of the Median Nerve And How They Change In Carpal Tunnel Syndrome1Leicester Royal Infirmary, Leicester, United Kingdom, 2University of Leeds, Leeds, United Kingdom
Synopsis
Carpal tunnel syndrome (CTS) is the most common compressive neuropathy worldwide. Compressed peripheral nerves exhibit distorted architecture, demyelination and perineurial fibrosis. Diffusion tensor imaging (DTI) generates proxy measures of nerve ‘health’ which are sensitive to myelination, axon diameter, fibre density and organisation. This meta-analysis included 32 studies of 2643 wrists, belonging to 1575 asymptomatic adults and 1068 patients with CTS. We summarise the normal FA (0·58 [95% CI 0·56-0·59]) and MD (1·138 x10-3 mm2/s [95% CI 1·101-1·174]) of the median nerve, then show that diffusion throughout the length of the median nerve is more isotropic in patients with CTS.
Introduction
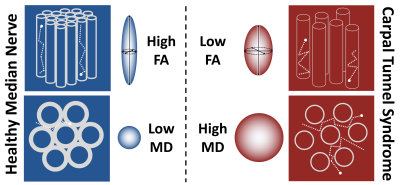
Carpal tunnel syndrome (CTS) is the most common compressive neuropathy, affecting 10 million people annually. Consequently, CTS is the most expensive upper extremity musculoskeletal disorder, costing the USA health system over $2 billion annually and employers up to $114,000 per incident.1 Compression of peripheral nerves leads to distortion of the axonal architecture, demyelination with or without poor remyelination, loss of the intrinsic vasculature and ultimately, fibrosis of the perineurial and epineurial connective tissue.2,3 Diffusion tensor imaging (DTI) characterises tissue microstructure and generates reproducible4–8 proxy measures of nerve ‘health’ which are sensitive to myelination, axon diameter, fibre density and organisation9–11 (Figure 1). This meta-analysis summarises the normal DTI values of the median nerve, and how they change in CTS.Methods
This review is registered with PROSPERO (CRD42020212378). It was designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews12 and has been authored in accordance with the PRISMA 2020 statement13. We included all studies which reported the findings of diffusion tensor magnetic resonance imaging of the median nerve, at the level of the wrist in asymptomatic adults or adults with CTS. The primary outcome was to determine the normal fractional anisotropy (FA) and mean diffusivity (MD) of the median nerve. Secondarily, we show how the FA and MD differ between asymptomatic adults and patients with CTS, and how these differences are independent of the acquisition methods. The raw data are available via the Open Science Framework (https://osf.io/vqwkp/). Data were pooled in random-effects models. Heterogeneity was quantified by I224 and explored using meta-regression. Restricted maximum likelihood was used to estimate the between-study variance (tau2), with the Knapp and Hartung modification.Results
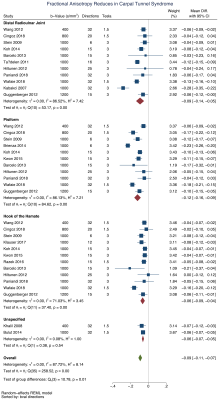
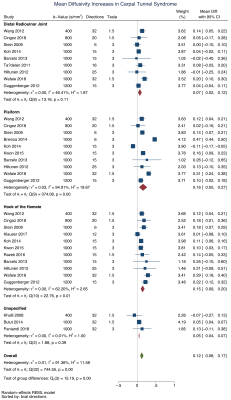
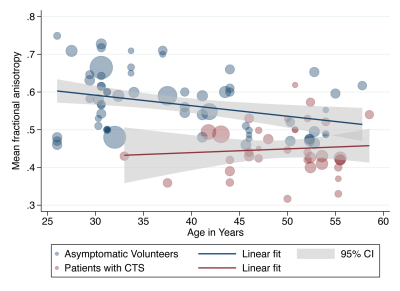
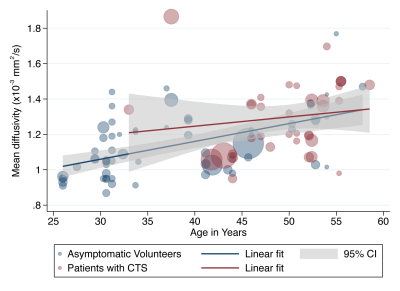
We included 32 studies of 2643 wrists, belonging to 1575 asymptomatic adults and 1068 patients with CTS. The normal FA was 0·58 (95% CI 0·56, 0·59) and the normal MD was 1·138 x10-3 mm2/s (95% CI 1·101, 1·174). Patients with CTS had a significantly lower FA than controls (mean difference 0·12 [95% CI 0·09, 0·16], Figure 2). Similarly, the median nerve of patients with CTS had a significantly higher mean diffusivity (mean difference 0·16 mm2/s x10-3 [95% CI 0·05, 0·27], Figure 3). Age was negatively associated with the FA in asymptomatic adults whereby each decade of life reduced the FA by approximately 0·003 (adjusted β -2·79 x10-3 [95% CI -4·78 x10-3, -8·12 x10-4]; I2 97%). However, there was no relationship between age and FA in patients with CTS (adjusted β 9·70 x10-4 [95% CI -2·89 x10-3, 4·83x10-3; I2 96%], Figure 4). There was no relationship between age and MD (Figure 5). There were no meaningful associations between experimental factors and DTI metrics.Conclusions
We provide summary estimates of the normal FA and MD of the median nerve in asymptomatic adults. Furthermore, we show that diffusion throughout the length of the median nerve becomes more isotropic in patients with CTS.Acknowledgements
No acknowledgement found.References
1. Milone MT, Karim A, Klifto CS, et al. Analysis of Expected Costs of Carpal Tunnel Syndrome Treatment Strategies. HAND. 2019;14(3):317–323.
2. NEARY D, EAMES RA. THE PATHOLOGY OF ULNAR NERVE COMPRESSION IN MAN. Neuropathol. Appl. Neurobiol. 1975;1(1):69–88.
3. Pham K, Gupta R. Understanding the mechanisms of entrapment neuropathies. Neurosurg. Focus. 2009;26(2):E7.
4. Nath V, Schilling KG, Parvathaneni P, et al. Tractography reproducibility challenge with empirical data (TraCED): The 2017 ISMRM diffusion study group challenge. J. Magn. Reson. Imaging. 2020;51(1):234–249.
5. Vavasour IM, Meyers SM, Mädler B, et al. Multicenter Measurements of T 1 Relaxation and Diffusion Tensor Imaging: Intra and Intersite Reproducibility. J. Neuroimaging. 2019;29(1):42–51.
6. Prohl AK, Scherrer B, Tomas-Fernandez X, et al. Reproducibility of Structural and Diffusion Tensor Imaging in the TACERN Multi-Center Study. Front. Integr. Neurosci. 2019;13(July):1–15.
7. Kimura M, Yabuuchi H, Matsumoto R, et al. The reproducibility of measurements using a standardization phantom for the evaluation of fractional anisotropy (FA) derived from diffusion tensor imaging (DTI). Magn. Reson. Mater. Phys. Biol. Med. 2019:15–19.
8. Cai LY, Yang Q, Kanakaraj P, et al. MASiVar: Multisite, multiscanner, and multisubject acquisitions for studying variability in diffusion weighted MRI. Magn. Reson. Med. 2021:mrm.28926.
9. Heckel A, Weiler M, Xia A, et al. Peripheral Nerve Diffusion Tensor Imaging: Assessment of Axon and Myelin Sheath Integrity. Plos One. 2015;10(6):e0130833.
10. Andersson G, Orädd G, Sultan F, et al. In vivo Diffusion Tensor Imaging, Diffusion Kurtosis Imaging, and Tractography of a Sciatic Nerve Injury Model in Rat at 9.4T. Sci. Rep. 2018;8(1):12911.
11. Friedrich P, Fraenz C, Schlüter C, et al. The Relationship between Axon Density, Myelination, and Fractional Anisotropy in the Human Corpus Callosum. Cereb. Cortex. 2020;30(4):2042–2056.
12. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collab. 2011.
13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10(1).
14. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558.
Figures