1378
Microstructural alteration of trigeminal nerve revealed by DTI and its correlation with vascular compression and pain1Department of Radiology, Liaocheng People's Hospital, Liaocheng, China, 2Department of Neurosurgery, Liaocheng People's Hospital, Liaocheng, China, 3Philips Healthcare, Shanghai, China
Synopsis
Classical Trigeminal Neuralgia (CTN) is mainly caused by vascular compression of the trigeminal nerve. We analyzed the correlation between the fractional anisotropy (FA) / apparent diffusion coefficient (ADC) values of the bilateral trigeminal nerves and the degree of neurovascular compression (NVC) for CTN patients. Results shown the FA value is negatively correlated with the degree of NVC on the symptomatic side. FA values was lower if there was NVC on the asymptomatic side compared with no NVC. The correlation between FA and visual analogue scale (VAS) scores indicates that FA was a potential MR indicator for predicting patient clinical symptoms.
Introduction
Classical trigeminal neuralgia (CTN) is characterized by an electroshock, intense and transient pain localized to the sensory supply areas of the trigeminal nerve, daily conversation, brushing or gently touch of the face can induce pain 1, which may affect the life of the patients seriously.Neurovascular compression (NVC) may also present on the asymptomatic side of some CTN patients. Previous results have shown that DTI can quantify microstructural changes caused by trigeminal nerve demyelination and axon membrane loss. 2-5
This study aims to explore the value of DTI in evaluating the microstructural alteration of the bilateral trigeminal nerves and its correlation with vascular compression and pain, which may provide more reliable information for the clinicians.
Materials and methods
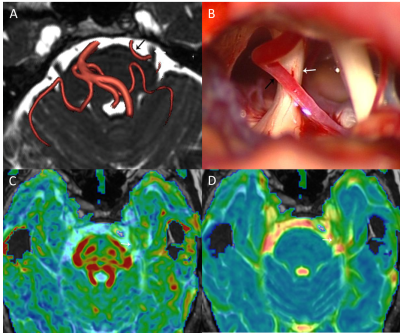
A total of 108 patients with CTN were enrolled in this study. All patients underwent three-dimensional time of flight (3D-TOF), three-dimensional fast imaging employing steady state acquisition (3D-FIESTA) and DTI scans. Patients were divided into 2 groups according to whether the asymptomatic side trigeminal nerve had NVC or not: group A with NVC and group B without NVC.The fractional anisotropy (FA) and apparent diffusion coefficient (ADC) of bilateral trigeminal nerves were measured at the GE AW4.6 workstation. Visual analogue scale (VAS) was used to evaluate the pain degree of the patients. The severity of NVC on the symptomatic side was classified as grade I, II and III by neurosurgeons according to the findings during microvascular decompression (MVD). Statistical analyses were performed using SPSS version 22.0.
Results
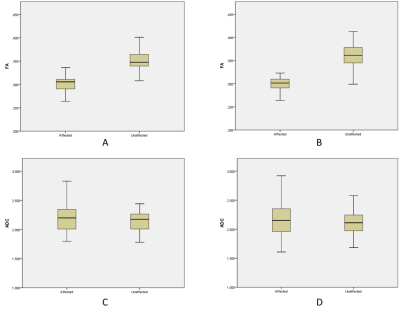
There were 32 cases in group A and 76 cases in group B.The FA values of trigeminal nerve in symptomatic side were significantly lower than those in asymptomatic side in group A (Fig 1.A, t = -15.262, P < 0.001) and group B (Fig 1.B, t = -22.629, P < 0.001). The mean ADC of trigeminal nerve in symptomatic side was higher than that in asymptomatic side, but the difference was not statistically significant (Fig 1.C and D, the t values were 1.536 and 1.716, respectively, P > 0.05).
The mean FA of trigeminal nerve in patients with NVC on the asymptomatic side was lower than that without NVC, and the mean ADC was higher than that without NVC, but the differences were not statistically significant (the t values were -1.856 and 0.479, respectively, P > 0.05).
36 patients (15 in group A and 21 in group B) were treated with MVD, with 12 cases of grade I, 17 cases of grade II, and 7 cases of grade III. The FA values of the trigeminal nerve were grade I: 0.309 ± 0.011, grade II: 0.295 ± 0.015, grade III: 0.286 ± 0.022, respectively, the difference was statistically significant (F = 5.172, P = 0.011). The ADC values were (2.156 ± 0.251) × 10-3, (2.144 ± 0.257) × 10-3, (2.223 ± 0.313) × 10-3 mm2/s, respectively, the difference was not statistically significant (F = 0.221,P > 0.05).
The FA of trigeminal nerve on the symptomatic side was negatively correlated with the degree of NVC and pain (the r values were -0.450 and -0.212, respectively, P < 0.05). ADC was not associated with the degree of NVC and pain (the r values were 0.054 and -0.025, respectively, P > 0.05).
Discussion
The decrease of FA values on symptomatic side may be related to microstructural changes such as nerve fiber demyelination, edema, and reduced axon density caused by vascular compression 6. Haller 7 believes that not all cases with NVC have TN symptoms. In this study, about 29.6% ((32 / 108)) patients have NVC for the trigeminal nerve in brain pool section on the asymptomatic side. The FA value of these trigeminal nerve with NVC have a decreasing trend compared with these without NVC, which further suggests that vascular compression may lead to a decrease in FA, and the absence of pain symptoms may be due to the mild degree of NVC.An analysis of symptomatic side trigeminal nerve in 36 patients with MVD showed a negative correlation between trigeminal nerve FA and the degree of NVC. This was consistent with Lutz’s studies 3, where the more obvious the nerve compression, the more obvious the demission and regeneration of the myelin, the more disordered the arrangement of the fiber bundles, and the lower FA values.
Conclusion
FA value is negatively correlated with NVC and VAS scores for CTN patients.Acknowledgements
Founded by Grant-in-aid for scientiêc research from the National Natural Science Foundation of China (No. 61976110)References
1. DI STEFANO G, MAARBJERG S, NURMIKKO T, et al. Triggering trigeminal neuralgia [J]. Cephalalgia, 2018, 38(6): 1049-1056.
2. WU M, QIU J, JIANG X F, et al. Diffusion tensor imaging reveals microstructural alteration of the trigeminal nerve root in classical trigeminal neuralgia without neurovascular compression and correlation with outcome after internal neurolysis[J]. 2020,37-44.
3. LUTZ J, THON N, STAHL R, et al. Microstructural alterations in trigeminal neuralgia determined by diffusion tensor imaging are independent of symptom duration, severity, and type of neurovascular conflict [J]. J Neurosurg, 2016, 124(3): 823-830.
4. HUNG P S, CHEN D Q, DAVIS K D, et al. Predicting pain relief: Use of pre-surgical trigeminal nerve diffusion metrics in trigeminal neuralgia [J]. Neuroimage Clin, 2017, 15(7): 10-18.
5. LIU Y, LI J, BUTZKUEVEN H, et al. Microstructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metrics [J]. Eur J Radiol, 2013, 82(5): 783-786.
6. CHEN S T, YANG J T, YEH M Y, et al. Using Diffusion Tensor Imaging to Evaluate Microstructural Changes and Outcomes after Radiofrequency Rhizotomy of Trigeminal Nerves in Patients with Trigeminal Neuralgia [J]. PLoS One, 2016, 11(12): e0167584.
7. HALLER S, ETIENNE L, KöVARI E, et al. Imaging of Neurovascular Compression Syndromes: Trigeminal Neuralgia, Hemifacial Spasm, Vestibular Paroxysmia, and Glossopharyngeal Neuralgia [J]. AJNR Am J Neuroradiol, 2016, 37(8): 1384-1392.
Figures