1375
Portable magnetic resonance based monitoring of MCA occlusion in an ovine sheep stroke model.1Victoria University of Wellington, Wellington, New Zealand, 2University of Otago, Wellington, New Zealand, 3The University of Auckland, Auckland, New Zealand, 4The University of Adelaide, Adelaide, Australia
Synopsis
A custom built single sided magnetic resonance system, generates an external region of homogeneous B0 = 0.2T field, a sweet spot, from which signal can be detected. The device is capable of relaxometry, diffusion and perfusion measurements and was used to continuously monitor progress of an MCA occlusion in a ovine sheep stroke model. The data from the device was compared to image data acquired on a clinical 3T MRI system.
Introduction
Following a stroke, time is brain, with more time passing conferring more brain tissue and function lost for the patient. As such, rapid diagnosis of stroke is critical for acute treatment, and relies on clinical imaging via Computed Tomography (CT) or Magnetic Resonance Imaging (MRI). These are large, costly to obtain and maintain, and require extensive infrastructure and expertise. This severely limits patient’s timely access to stroke diagnosis and therefore acute treatment. Especially in rural areas and developing countries, this lack of access contributes to higher rates of death and disability in this time sensitive disorder. [1] Decentralisation, and other efforts to reduce diagnosis/door to needle time or increase treatment windows, are effective at increasing acute treatment rates and improving stroke outcomes. However, these still rely on conventional imaging paradigms, ultimately limiting their applicability. Providing an accessible, affordable, and portable alternate diagnostic paradigm could markedly relieve the burden of delayed or inaccessible treatment, particularly in the most vulnerable or remote populations. Our group has developed a single-sided, 20kg, low-field, magnetic resonance (MR)-based device for the rapid detection of stroke pathologies in vivo, which can also be applied outside of the hospital setting [4].Methods
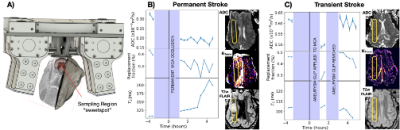
The ovine stroke model we used has been developed to study the pathophysiological changes that occur following ischemic stroke and to aid the investigation of new therapies [2,3]. The protocol for this study was approved by the Animal Ethics Committee of the University of Adelaide. The cohort of 11 merino sheep was divided into two groups: 6 underwent permanent occlusion surgery, and 5 underwent temporary occlusion surgery. Each animal was anaesthetised and we measured a baseline/”healthy” measurement using our device. Following measurements, access to the brain was obtained by craniotomy. The Middle Cerebral Artery was located and occluded using either electrocauterization for the permanent animals, or a mini aneurysm clip for the temporary animals. The bone was reinstated, and access closed up before measurements were made again. The animals were monitored using a portable MR system for a total period of 4 hours. In the temporary group, after a 2 hour period, the brain was accessed again and the clip was removed, opening the MCA, before closing the craniotomy and continuing to monitor with the system. Following the low field MR monitoring, the animals were immediately taken to the MRI suite (3T Siemens Skyra). For this study, the MRI scans included T1-weighted anatomical, T2-weighted FLAIR (SPACE), DCE with Gadobutrol contrast (TWIST), MRA, T1 mapping (VFA VIBE) and DWI. The images provide a true value for comparison to our device which measures T2, ADC and perfusion.Results
Data from the permanent stroke model showed a decrease in apparent diffusion coefficient (ADC), tissue perfusion (blood replacement fraction), and an increase in T2, after occlusion which correlates well with the MRI images (Figure 1b). In contrast, data from the transient stroke model showed a decrease in ADC following the occlusion, along with a decrease in tissue perfusion (replacement fraction) and little change in T2. After the clip is removed, both ADC and perfusion recover to close to their original value, and T2 is decreased slightly. This recovery correlates well with the MRI images, which show no difference between the two hemispheres. This agrees with previous studies of transient ischemia [5,6].Conclusion
The ability to monitor brain tissue outside of an imaging suite is a potential game-changer, allowing clinicians to react sooner to instigate life-saving treatments. While this approach departs from using conventional Magnetic Resonance Imaging to track stroke, the simplicity of the device allows it to be portable and thus more ubiquitous, extending rather than replacing MRI.Acknowledgements
This project is funded by the New Zealand Ministry of Business Innovation and Employment. We would also like to acknowledge the facilities and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at LARIF, SAHMRI.References
[1] S. S. Bhat et al., “Low-Field MRI of Stroke: Challenges and Opportunities,” Journal of Magnetic Resonance Imaging, vol. 54 no. 2, 2021, p. 372-390, doi: https://doi.org/10.1002/jmri.27324.
[2] A. J. Wells et al., “A Surgical Model of Permanent and Transient Middle Cerebral Artery Stroke in the Sheep,” PLOS ONE, vol. 7, no. 7, p. e42157, Jul. 2012, doi: 10.1371/journal.pone.0042157.
[3] A. J. Sorby-Adams et al., “Determining the Temporal Profile of Intracranial Pressure Changes Following Transient Stroke in an Ovine Model,” Frontiers in Neuroscience, vol. 13, p. 587, 2019, doi: 10.3389/fnins.2019.00587.
[4] D. Thomas et al., “Single sided magnet system for relaxometry and diffusion measurement,” in Proceedings of the 28th Annual Meeting of ISMRM, Jul. 2020, Abstract #1255.
[5] F. A. van Dorsten et al., “Dynamic changes of ADC, perfusion, and NMR relaxation parameters in transient focal ischemia of rat brain,” Magnetic Resonance in Medicine, vol. 47, no. 1, pp. 97–104, 2002, doi: 10.1002/mrm.10021.
[6] M. E. Moseley et al., “Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and T2-weighted MRI and spectroscopy,” Magnetic Resonance in Medicine, vol. 14, no. 2, pp. 330–346, 1990, doi: 10.1002/mrm.1910140218.
Figures