1370
Diffusion Imaging comparison of high-performance Gradient system (MAGNUS) with clinical MR system.1Radiology and Radiological Sciences, Uniform Services University of the Health Sciences, Bethesda, MD, United States, 2GE Global Research, Niskayuna, NY, United States, 3GE Healthcare, Niskayuna, NY, United States, 4Walter Reed National Military Medical Center, Bethesda, MD, United States, 5Uniform Services University of the Health Sciences, Bethesda, MD, United States
Synopsis
Comparisons of the multi-shell DWI is described for the USU/GE MAGNUS 3T head-only gradient system and a clinical whole-body GE MR750 3T magnet system. The advantages of high-performance gradients are shown in analysis outcomes of dMRI metrics. Direct comparison is made between identical diffusion pulse sequences and system optimized sequences for each platform. The shorter TE and high-gradient strength of MAGNUS enhance SNR and diffusion imaging response.
Introduction
The prototype microstructure anatomy gradient for neuroimaging with ultrafast scanning (MAGNUS) system generates magnetic field gradients of 200mT/m, and 500 T/m/s compared to a clinical whole-body MRI (50 mT/m, 200 T/m/s).1 These gradient advances allow for more rapid image acquisition with reduced EPI echo spacing, diffusion TE, reduced distortion and blurring while reporting higher PNS thresholds compared to whole-body gradient systems.2 Higher spatial and temporal resolution can be targeted with shorter TE times and stronger diffusion weighting, enabling better narrow-pulse approximation and the microstructure study with a more relevant parameter space.3 These developments have enabled advanced neuroimaging applications with simplified biophysical modeling leading to a more robust interpretation of in vivo architecture.4,5We compare the diffusion encoding performance of the clinical whole-body GE MR750 system and the MAGNUS system. The Head-Health Initiative (HHI-NFL)6,7 traumatic brain imaging protocol was used as a standard-of-reference on the clinical whole-body 3T and an optimized version was used on the MAGNUS system taking full advantage of the gradient performance to improve diffusion imaging.
Methods
MR Acquisition: Four volunteers were scanned under IRB-approval with written informed consent, on the MAGNUS 3T MR system which is a standard 3T MR system (GE SIGNA MR750, Waukesha,WI) retrofitted with a head-only MAGNUS gradient sub-system capable of a peak gradient amplitude of 200 mT/m with a slew rate of 500 T/m/s. The clinical whole-body 3T MR system (GE SIGNA MR750) has a standard gradient capable of a peak gradient amplitude of 50 mT/m with a 200 T/m/s slew rate. All imaging was performed using a 32-channel phased-array receive head coil (NOVA Medical, Wilmington,MA,USA).Multi-shell diffusion acquisitions were performed with 147 directions identical to the HHI-NFL protocol on the clinical whole-body system (3 shell, bmax=2,800 s/mm2), and 125 directions on the MAGNUS system (3 shell, bmax=4,000 s/mm2). The gradient directions were uniformly sampled over a sphere with a Coulomb electrostatic repulsion algorithm.8 Other parameters on the clinical whole-body MR: EPI echo spacing 632 µs, TE/TR=80.1/5000 ms, 2.2-mm isotropic resolution, 1 signal average (NEX=1) R=2 in-plane acceleration. Diffusion scan parameters on MAGNUS were: EPI echo-spacing 548 µs, TE/TR = 44.6/5000 ms, 1.5-mm isotropic resolution, 1 signal average (NEX=1), R=2 in-plane acceleration. Each acquisition was ~10 min. Volunteers were scanned on both system the same day.
Processing: For both systems identical post processing algorithms were utilized. Diffusion-weighted images were corrected for gradient non-linearity, eddy-current distortion, bulk motion and susceptibility using a custom image processing pipeline.9,10 Generalized Spherical deconvolution (GenSD) denoising was applied to both acquisitions.11,12 Diffusion and kurtosis tensors were fitted using a non-negativity constrained least-squares approach to compute diffusion and kurtosis tensor metrics. Fiber orientation distribution functions (fODFs) and anatomically-constrained probabilistic tractograms were generated for all datasets using MRtrix3.13
Analysis: First, to remove spatial resolution as a confound in quantitative comparisons, MAGNUS data was resampled (in-plane: resampling k-space and through-plane: 1D box kernel on raw data in z-direction) to match the spatial resolution for acquisitions on the clinical system. Second, data was evaluated with and without denoising using GenSD to assess the impact of the performance of the signal model. Further, FA and kurtosis metrics were quantitatively compared with histogram analysis.
Results and Discussion
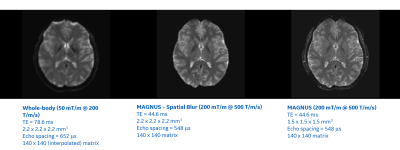
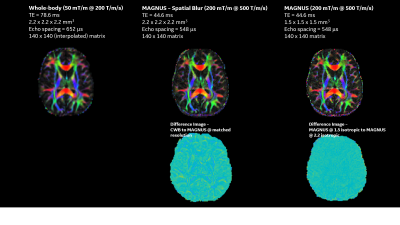
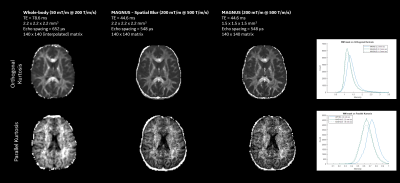
Shorter TE and tighter EPI echo spacing on MAGNUS reduced susceptibility artifacts and geometric distortion with higher slew rate while reducing diffusion blurring in computed fiber tracks as compared to the clinical 3T MR system.T2-weighted Figure 1 shows b=0 images highlight the increased resolution conspicuity and SNR available from the reduced echo-times, improved image clarity and reduced distortion with MAGNUS, even for spatially matched images highlighting feature retention in the absence of T2* blurring (Figure 1). Diffusion (Figure 2) and Kurtosis (Figure 3) metrics highlight increased conspicuity and diffusion encoded detail with acquisitions using the MAGNUS platform. Increased conspicuity in images is further highlighted via difference maps, where at the same spatial resolution MAGNUS data highlights significantly improved acuity in gray and white matter interfacial regions (Figure 2). Histogram analysis of all white matter (WM) voxels for orthogonal and parallel kurtosis highlights, higher mean kurtosis with a larger FWHM for the clinical scanner compared to MAGNUS (not a function of spatial resolution). Striking fine white matter structural detail is noticeable with the parallel kurtosis maps (Figure 3). Figure 2 and 3 show DTI results with color FA maps and orthogonal kurtosis. The clarity of the MAGNUS-optimized protocol demonstrates the advantages of shorter diffusion encoding, reduced TE, and shorter EPI echo spacing. Figures 4 and 5 demonstrate the downstream processing results that are available using the advances in gradient performance.
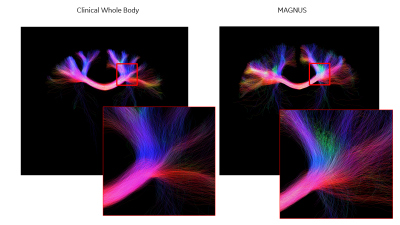
Comparisons for fiber ODFs are presented for the corona radiata highlight crossing fiber detail and superior cortical areas to highlight cortical connectivity (Figure 4). The sharper fODFs from MAGNUS directly translates into any tractography analysis where for the same region fine bundle detail is evident (Figure 5).
Conclusion
Experimental results highlight superior performance with MAGNUS compared to clinical whole body acquisitions, in quantitative metrics, diffusion encoding, and microstructural detail. MAGNUS achieved 104 μs shorter effective echo-spacing and ~2x shorter echo-time. This resolves finer structures for better determination of white matter changes in disease processes with increased sensitivity in encoding diffusion probability distribution.Acknowledgements
Grant funding from NIH U01EB028976, NIH U01EB024450, CDMRP W81XWH-16-2-0054.
The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University.
References
1. Foo, T. K. F. et al. Magn Reson Med 83, 2356–2369 (2020).2. Tan, E. T. et al. Magn Reson Med 83, 352–366 (2020)
3. Caprihan, A. et al. J Mag Res 118, 94–102 (1996).
4. Tan, E. T. et al. Magn Reson Med 84, 950–965 (2020).
5. Zhu, A. et al. Proceedings of the 29th Meeting ISMRM, 3639 (2021)
6. Marinelli, L. et al. Neurology 86, (2016).
7. Li, C.-X. et al. Quant Imaging Med Surg 10, 824–834 (2020).
8. Dk, J. et al. Magn Reson Med (1999) vol. 42
9. Tan, E. T. et al. JMRI 38, 448–453 (2013).
10. Bhushan, C. et al. Neuroimage 115, 269–280 (2015).
11. Sperl, J.A. et al. Magn Reson Med 78, 2428-2438 (2017)
12. Abad, N. et al. Proceedings for the 29th meeting ISMRM, 2476 (2021)
13. Tournier, J-D. et al. Neuroimage 202, 116137 (2019).
Figures