1367
Short-TE diffusion-MRI by combining strong gradients with ultrasonic readout1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Electromechanics & Power Electronics, Eindhoven University of Technology, Eindhoven, Netherlands, 3CUBRIC, School of Physics and Astronomy, Cardiff University, Cardiff, United Kingdom, 4Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 5Prodrive Technologies B.V., Son, Netherlands
Synopsis
For high-resolution diffusion-MRI, strong gradients combined with fast readout are desired. In this study, we propose a gradient system architecture for such strong gradients and fast readouts without nerve stimulation by implementing a filter topology that allows for both low and high frequency gradient waveforms. Two gradient inserts with different specifications are realized and two proposed modulation strategies are implemented in the amplifier firmware. Field measurements demonstrated the desired dual-mode capability of the system. With one gradient insert, a gradient amplitude of 300 mT/m and slew rates well above 10.000 T/m/s can be reached with only 500A&330V as amplifier output.
Purpose
Diffusion-weighted MRI can probe the architectural configuration of tissue microstructure, yet requires very strong gradients, particularly when considering tissues with short T2 relaxation times. While whole body designs that facilitate gradients up to 300 mT/m have been developed 1, they suffer from relatively slow ramp times that avoid peripheral nerve and cardiac stimulation, and require a significant physical footprint. Moreover, as these designs are specifically targeted towards high field strengths, they are suboptimal during the echo planar imaging (EPI) readout. In this paper we propose a new gradient system architecture, for which typical gradient amplifier hardware can be employed. The proposed approach enables seamless switching between two modes, one for strong gradients and one for ultrasonic readout. The method is experimentally verified, demonstrating that a diffusion gradient followed by a 20 kHz readout is feasible and significantly reduces the sound pressure levels and nerve stimulation associated with an EPI readout.Methods
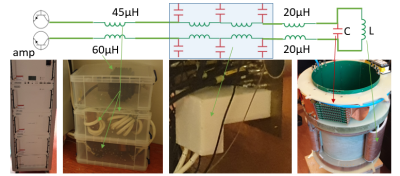
The proposed gradient system architecture, shown in Figure 1, consists of a typical gradient amplifier and a modified, distributed output filter. Additionally, the gradient amplifier modulation scheme is adapted to cater two modes of operation: one using conventional pulse-width modulation (PWM) with reference tracking, and one fixed frequency modified sine wave operation with amplitude control, to allow strong gradients and ultrasonic readout respectively.Combining a resonant filter with the gradient head coil results in a third-order LCL filter. By using a capacitor in parallel with the system's gradient coil, the filter is capable of handling DC current, which enables low frequency gradient waveforms. Additionally, the filter is tuned such that the resonance frequency is around 20 kHz to allow an ultrasonic EPI readout, according to
$$ \omega_ {res} = \sqrt{\frac{L_1 + L_1}{L_1L_2C}} .$$
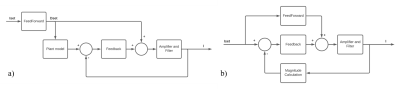
By utilizing the amplifying characteristic of the resonance, the high slew rates (up to 10.000 T/m/s) for the 20 kHz operation are provided. Additionally, a hybrid feed-forward and feedback control method is proposed that significantly improves the performance of the overall system, as depicted in Figure 2. The first gradient coil was paired with an off-the-shelf gradient amplifier (Prodrive, Netherlands), Here, the proposed modulation strategies were implemented in the amplifier firmware to enable the amplifier to produce high frequency waveforms as well as low frequency gradient waveforms. The proposed gradient architecture was evaluated by measuring the gradient amplitude with a field camera (Skope, Switzerland). During these measurements, the setup was controlled by an external waveform generator that was triggered by the MR-system (Philips Achieva 7T, Netherlands). As a proof-of-principle and to ensure that the signals of the field camera do not become completely dephased by the typical long diffusion pulses, we used a relatively short “diffusion” pulse (1ms), followed by the 20 kHz EPI readout (10ms).
Results
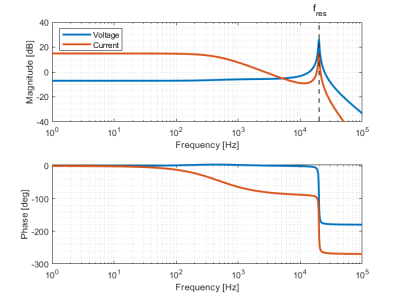
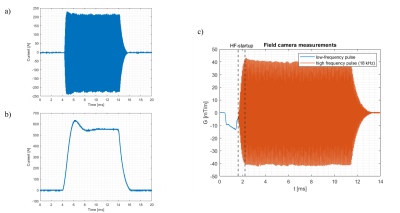
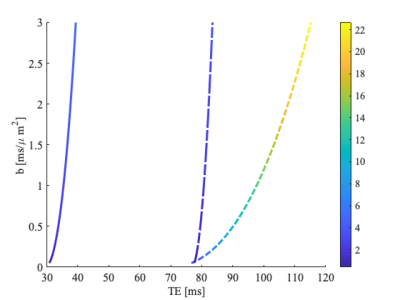
Two insert gradients were built, the first one with inductances of the Y- and Z gradient of 30.46 and 38.24 μH and efficiencies of 0.095 and 0.3 mT/m/A, and the second insert gradient with inductances of the Y- and Z gradient of 61.07 and 261.65 μH and efficiencies of 0.095 and 0.60 mT/m/A respectively. The frequency response function of the designed LCL filter for the input voltage to gradient insert voltage and current is shown in Figure 3. The voltage over the insert gradient is attenuated by a factor two for the low frequency region (below 1 kHz) and amplified at the resonance frequency of 20 kHz by a factor 21. Figure 4ab show the amplifier current for a diffusion gradient and ultrasonic readout, respectively. These measurements demonstrate that both the low frequency diffusion gradient as well as the high frequency readout currents can be provided to the gradient coil. Despite the high amplification factor of the resonant coil, we could reach steady-state of the silent EPI train in 0.5 ms due to the proposed control scheme. Figure 4c shows the measured field for a low diffusion pulse followed by the silent readout of 40 mT/m. Figure 5 presents the TE achievable for a given diffusion weighting (characterized by the b-value), leveraging the strong gradients and ultrasonic readout of the insert compared to state-of-the-art gradient setups.Discussion & Conclusion
We have demonstrated a setup which operates the gradient system in dual-mode, to generate a low frequency gradient pulse followed by an ultrasonic readout. The proposed control algorithm of the ultrasonic readout reduced the rise time of the field amplitude significantly, as can be seen in Figure 5 by comparing the HF-startup time with the damped oscillation time at the end of the sequence (0.5 and 2.0 ms respectively). The control algorithm of the diffusion pulse can be further improved by tuning the control parameters. Depending on the properties of the gradient insert and the used amplifier, gradient amplitude of 300 mT/m at 500 A can be obtained for the Z-gradient, and both Y and Z gradients could be driven well above a 10.000 T/m/s during the silent EPI readout. The combination of wave encoding and EPI readout opens up avenues for high accelerations and resolution while reducing g-factor and artifacts 2.Acknowledgements
No acknowledgement found.References
1) D. K. Jones, D. C. Alexander, R. Bowtell, M. Cercignani, F. Dell’Acqua, D. J. McHugh, K. L. Miller, M.Palombo, G. J. M. Parker, U. S. Rudrapatna, and C. M. W. Tax, “Microstructural imaging of the human brain with a ‘super-scanner’: 10 key advantages of ultra-strong gradients for diffusion MRI,” NeuroImage,vol. 182, no. May, pp. 8–38, 2018.2)
2) J. Cho, C. Liao, Z. Zhang, W.-c. Lo, J. Xu, O. Beker, K. Setsompop, and B. Bilgic, “Highly Accelerated EPI with Wave Encoding and Multi-shot Simultaneous Multi Slice Imaging,”Proc. Int. Soc. Magn. Reson.Med., p. submitted, 2020.
Figures