1363
1H-31P Cross-polarization: A new frontier to study myelin in white matter1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries (ICORD), University of British Columbia, Vancouver, BC, Canada, 3Radiology, University of British Columbia, Vancouver, BC, Canada, 4UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 5Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada
Synopsis
Phosphorus (31P) nuclear magnetic resonance spectroscopy (NMR) has the potential to play a valuable role in the characterization of myelin due to the concentration of 31P present in myelin phospholipids. This study demonstrates the feasibility of detecting 31P through cross polarization (CP) and magnetization transfer (MT) from hydrogen in preserved murine spinal cord and fresh porcine brain. The results demonstrate the detection of myelin phospholipids through these NMR techniques and provide compelling proof of concept for the potential applicability of MT-CP in 31P MRI for non-ambiguous myelin characterization in vivo.
Introduction
The development of methods to detect and characterize the health of myelin in white matter tissue is an active area of research. Methods such as magnetization transfer (MT)1,2, myelin water fraction3,4, diffusion tensor imaging5, T1 contrast6,7 and inhomogeneous magnetization transfer8 are all used to study white matter, and the relative merits of these and other techniques continue to be debated in the literature.Solid-state 31P spectroscopy, which can measure phosphorus in lipids, is expected to be extremely specific to myelin not only because of the abundance of lipids in myelin, but also because of the fact that phosphorus is more abundant in myelin lipids than in other lipids.9 We introduce a new avenue of research to study white matter tissue based upon the cross-polarization (CP) technique which is commonly used in solid-state NMR.10 We present initial results exploring the feasibility of CP in brain and spinal cord white matter tissue samples and demonstrate the successful two-stage transfer of nuclear spin polarization first from aqueous 1H to semi-solid lipid protons via MT, followed by CP to detect the 31P NMR spectrum.
Methods
Two different types of central nervous system tissue were investigated: preserved cervical murine spinal cord (70mg), and fresh porcine brain (250mg). One male Sprague-Dawley rat, was euthanized by intraperitoneal injection of 5% chloral hydrate while under isoflurane anesthesia. The rat was perfused via intra-cardial perfusion, with 250mL of phosphate buffered saline followed by 500mL of 4% paraformaldehyde. The spinal cord was removed, and stored in paraformaldehyde solution. Brain tissue used was white matter extracted from the pons of a Yucatan miniature pig (Premier Biosource) immediately after death. Experiments were completed within 48h of the time of death.NMR spectroscopy experiments were performed using a lab-built solid-state spectrometer11 with an 8.4 T (360 MHz 1H frequency, 146 MHz 31P) magnetic field, and a horizontal-coil double-resonant probe.
Data acquisition
Pulse sequences are shown in Fig. 1. A decoupled 31P Bloch decay sequence is shown in Fig. 1A. In addition to the vanilla CP sequence in Fig 1B, modified sequences to perform a 2D WIdeline SEparation (WISE)12 experiment (Fig. 1C) and a Goldman-Shen (GS)13 filtered MT-CP experiment (Fig. 1D) are shown. A recycle delay of 3.5s was used in all experiments. RF field strengths of 27 kHz were used on both channels with a CP contact time of 3-5ms.
Results
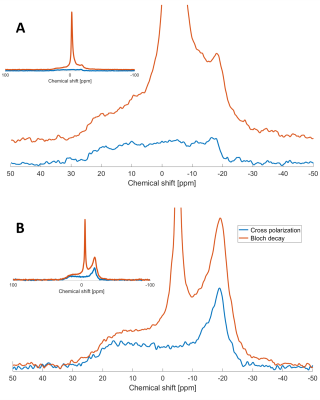
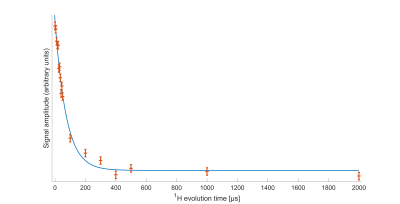
Decoupled Bloch decay spectra from the spinal cord (Fig 2A) show a large narrow 31P peak in addition to the ~6kHz wide powder pattern14 from the lipids, due to both phosphorus in isotropically reorienting aqueous metabolites as well as the buffer used in sample preservation. The corresponding aqueous contribution for the fresh brain white matter tissue is seen in Fig. 2B with considerably lower relative amplitude. The aqueous peaks are absent in the CP spectra.The WISE-CP experiment (Fig. 3) shows that the 1H magnetization responsible for the CP signals decays with T2 of 73µs.
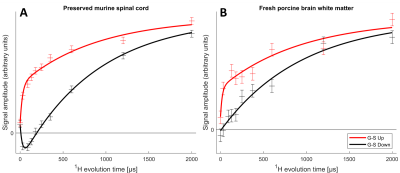
The MT-CP experiments (Fig. 4), which first dephase the semi-solid 1H magnetization then place the aqueous 1H magnetization either parallel or anti-parallel to B0 before a variable MT time and CP, show that MT from the aqueous 1H can be observed in the 31P spectra. A bi-exponential fit to the murine spinal cord MT data reveals an aqueous-lipid cross-relaxation time constant of 27ms for murine spinal cord and an approximation of 20ms for porcine brain.
Discussion
The absence of aqueous peaks in the CP spectra confirm that these signals reflect only those 31P in semi-solid environments. The dip in the center of the fresh porcine brain sample CP spectrum suggests that CP efficiency is reduced for lipids aligned near the magic angle15, where intra-molecular dipolar couplings that are partially averaged by the motion of lipid molecules within bilayers would be expected to vanish16.The WISE experiment shows conclusively that it is 1H magnetization from the semi-solid component (having a 1H linewidth of ~4.3kHz) that participates in CP. The MT-CP experiment shows that this semi-solid 1H magnetization exchanges with aqueous magnetization on a time scale of 20-30ms.
Detection of myelin through the 31P nuclei has the potential to be much more specific than 1H-only based techniques. Additional features of 31P spectroscopy, such as details of lineshape17 and relaxation are sensitive to structure and dynamics of the membrane and may be useful indicators of myelin health16. An obvious challenge with solid-state 31P NMR in vivo arises from the breadth of the NMR spectra, ~40ppm, which severely limits the sensitivity. Ultimately, our goal is to detect the solid-state 31P spins through aqueous 1H, reversing the MT transfer observed in the MT-CP experiment demonstrated here. A second challenge is the high RF field strengths associated with CP experiments. We plan to use lower amplitude and adiabatic pulses to explore the feasibility of CP experiments in vivo.
Conclusion
These results demonstrate proof-of-principle for using CP combined with MT to transfer magnetization between aqueous 1H nuclei and non-aqueous 31P nuclei in white matter. The concepts shown in this study open a new window for the study of myelin and have potential for translation to clinical applications.Acknowledgements
This work was conducted on the traditional, ancestral, and unceded territories of Coast Salish Peoples, including the territories of the xwməθkwəy̓əm (Musqueam), Skwxwú7mesh (Squamish), Stó:lō and Səl̓ílwətaʔ/Selilwitulh (Tsleil- Waututh) Nations.References
1. Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: A review. NMR Biomed. 2001;14(2):57-64. doi:10.1002/nbm.683
2. Stanisz, G.J., Kecojevic, A., Bronskill M.J., Henkelman RM. Characterizing White Matter With Magnetization Transfer and T2. Magn Reson Med. 1999;42:1128-1136. doi:10.2307/2316017
3. Mackay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31(6):673-677. doi:10.1002/mrm.1910310614
4. Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DKB, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37(1):34-43. doi:10.1002/mrm.1910370107
5. Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13(4):534-546. doi:10.1002/jmri.1076
6. Labadie C, Lee JH, Rooney WD, et al. Myelin water mapping by spatially regularized longitudinal relaxographic imaging at high magnetic fields. Magn Reson Med. 2014;71(1):375-387. doi:10.1002/mrm.24670
7. Se-Hong Oh, Michel Bilello, Matthew Schindler, Clyde E. Markowitz, John A. Detre and JL. Direct Visualization of Short Transverse Relaxation Time Component (ViSTa). Neuroimage. 2013;83. doi:10.1016/j.neuroimage.2013.06.047.Direct
8. Manning AP, Chang KL, MacKay AL, Michal CA. The physical mechanism of “inhomogeneous” magnetization transfer MRI. J Magn Reson. 2017;274:125-136. doi:10.1016/j.jmr.2016.11.013
9. Oliveira MJ, Águas AP. High concentration of phosphorus is a distinctive feature of myelin. An X-Ray elemental microanalysis study using freeze-fracture scanning electron microscopy of rat sciatic nerve. Microsc Res Tech. 2015;78(7):537-539. doi:10.1002/jemt.22506
10. Pines A, Gibby MG, Waugh JS. Proton-enhanced nuclear induction spectroscopy. a method for high resolution nmr of dilute spins in solids. J Chem Phys. 1972;56(4):1776-1777. doi:10.1063/1.1677439
11. Michal CA, Broughton K, Hansen E. A high performance digital receiver for home-built nuclear magnetic resonance spectrometers. Rev Sci Instrum. 2002;73(2 I):453. doi:10.1063/1.1433950
12. Schmidt-Rohr KR, Clause J, Spiess HW. Correlation of Structure, Mobility, and Morphological Information in Heterogeneous Polymer Materials by Two-Dimensional Wideline-Separation NMR Spectroscopy. Macromolecules. 1992;25(12):3273-3277. doi:10.1021/ma00038a037
13. Goldman M, Shen L. Spin-Spin Relaxation in LaF3. Phys Rev. 1966;144(1):321-331. https://link.aps.org/doi/10.1103/PhysRev.144.321
14. Filippov A V., Khakimov AM, Munavirov B V. 31P NMR Studies of Phospholipids. Vol 85. 1st ed. Elsevier Ltd.; 2015. doi:10.1016/bs.arnmr.2014.12.001
15. Molugu TR, Lee S, Brown MF. Concepts and Methods of Solid-State NMR Spectroscopy Applied to Biomembranes. Chem Rev. 2017;117(19):12087-12132. doi:10.1021/acs.chemrev.6b00619
16. Hong M, Schmidt-Rohr K, Nanz D. Study of phospholipid structure by 1H, 13C, and 31P dipolar couplings from two-dimensional NMR. Biophys J. 1995;69(5):1939-1950. doi:10.1016/S0006-3495(95)80064-0
17. Pettegrew JW, Klunk WE, Kanal E, Panchalingam K, McClure RJ. Changes in brain membrane phospholipid and high-energy phosphate metabolism precede dementia. Neurobiol Aging. 1995;16(6):973-975. doi:10.1016/0197-4580(95)02017-9
Figures
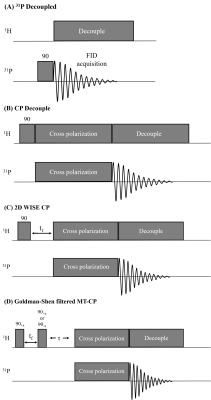
Pulse sequences. (A) Bloch-decay sequence with proton decoupling. (B) The basic CP pulse sequence consists of a 1H π/2 pulse followed by a contact-time that transfers 1H polarization to 31P via dipolar couplings. (C) 2D-WISE sequence where a 1H evolution time (t1) is inserted after the π/2 pulse, allowing detection of the 1H FID. (D) A GS filter (tf=200-300us) prior to CP dephases the semi-solid 1H, placing the aqueous 1H magnetization along or perpendicular to B0, allowing MT during τ.