1362
Optimization of a Phase-Cycled bSSFP for Improved SNR-efficiency in Fluorine-19 MRI at 3T1Medical physics, University of Wisconsin Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 3Radiology, University of Wisconsin, Madison, Madison, WI, United States, 4Radiology, University of Iowa, Iowa City, WI, United States
Synopsis
The focus of this work is on the optimization of fluorine-19 enabled SPGR, bSSFP, and phase cycled bSSFP (bSSFP-C) pulse sequence parameters to improve signal acquisition efficiency for perfluoropolyether (PFPE) at 3T with a multi-channel coil. Bloch simulations and subsequent experimental validation were performed to determine the parameters to maximize the SNR-efficiency for each sequence. Acquisitions of the optimized SPGR, bSSFP, and bSSFP-C were then assessed for their achieved SNR-efficiency. bSSFP and bSSFP-C demonstrated increased sensitivity compared to SPGR. Feasibility of bSSFP-C with parallel imaging demonstrated the ability to reduce scan times by 2-fold without compromising the qualitative image quality.
Purpose
Fluorine-19 MRI (19F-MRI) is a noninvasive and nonionizing imaging platform that has been used to track and monitor adoptively transferred immune cells days after injection, in vivo1. While the utility of 19F-MRI has been demonstrated numerously on small animal scanners, only a handful of studies have demonstrated its utility on clinical scanners2–5. Of these clinically oriented studies, many utilized adapted mouse coils or single-channel surface coils. 19F-MRI is often concerned with acquisition efficiency due to the low intrinsic spin-density that can be achieved in vivo, which has led to the use of highly signal efficient sequences, such as balanced steady-state free precession (bSSFP); however, SSFP sequences are prone to off-resonance banding artifacts. Given the challenges associated with clinical multinuclear MRI, field inhomogeneities may preclude the use of SSFP on a clinical multichannel coil. One technique to address this concern is to utilize a phase-cycling approach.6,7 In the work presented here, the purpose was to: optimize and validate parameter selection for perfluoropolyether (PFPE), a commonly used cell tracking probe, to improve SNR-efficiency (SNReff) for SPGR, bSSFP, and phase cycled bSSFP acquisitions, assess the achieved sensitivity, and lastly, demonstrate the feasibility of parallel imaging to further reduce time constraints.Methods
T1 and T2 values were determined via spectroscopic inversion recovery FID and spin echo sequences. Bloch simulations were performed on MATLAB 2020a (Mathworks, Natick, MA) for SPGR and bSSFP acquisitions using the previously measured T1 and T2 times of 424 and 165 ms for PFPE to determine the optimal flip angle (FA), TR, and receiver bandwidth (rBW) that will maximize the theoretical SNReff. All acquisitions were performed at 3T (MR 750 HD, GE Healthcare) with agar phantoms (2% by weight, KCl: 30mM) within the coil to ensure adequate loading and reproducible coil spacing. A flip angle calibration was performed by manual adjusting the transmit gain for a 90°-pulse, then attenuating to our desired FA. Validation of these theoretical optimal parameters, like the Ernst angle, was performed using multi-nuclear versions of SPGR, FIESTA, and FIESTA-C product pulse sequences on an 8-channel torso coil (MRI.TOOLS, Berlin, Germany) with a vial of concentrated PFPE in the FOV. Sequences were prescribed at the optimal parameters for a 10-minute acquisition of 4 vials of descending concentrations of PFPE in agar (2% w/w) placed within the FOV. The resulting SNReff and its concentration-normalized value, hereafter the sensitivity (S), were defined as:$$ SNR_{eff}=\frac{\mu}{\sigma\sqrt{TR\cdot\rho}}=\frac{SNR}{\sqrt{TR\cdot\rho}}\ \ \ \ \ \ \ [\mathrm{\mathrm{m}}\mathrm{s}^{\mathrm{-0.5}}] \tag{Eq. 1a}$$
$$ S=\ \frac{SNR_{eff}}{C_{PFPE}\cdot V_{vox}}\ \ \ \ \ [\mathrm{\mathrm{m}}\mathrm{s}^{\mathrm{-0.5}}\mathrm{\mu mo}\mathrm{l}^{\mathrm{-1}}] \tag{Eq. 1b}$$
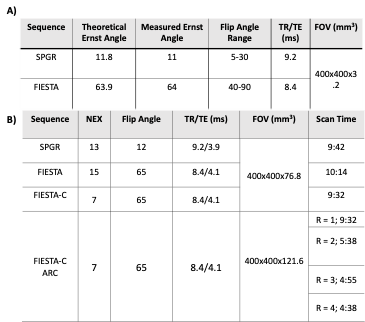
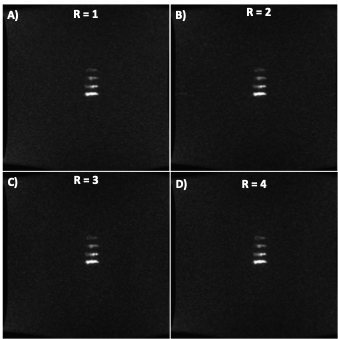
where μ is the mean signal, σ is the corrected noise standard deviation, ρ is the number of phase cycles, CPFPE is the concentration of PFPE in the respective vials, and Vvox is the voxel volume. SNReff and sensitivity measurements for each vial were used as the figure of merit and calculated for each sequence and set of parameters (Eq 1a and 1b) and compared via one-way ANOVA to determine relative performance. ARC parallel imaging techniques were implemented on the 10-minute FIESTA-C acquisition where nominal acceleration factors (R) between 1-4 were used to investigate the effects of increasing acceleration on image quality and overall vial detection. All relevant parameters for the sequences can be seen in Figure 1. The mean signal and noise were found by manually drawing ROIs within the boundaries of each detectable PFPE vial and placing large ROIs within regions of no signal and correcting for the magnitude bias seen in multi-channel coils.
Results
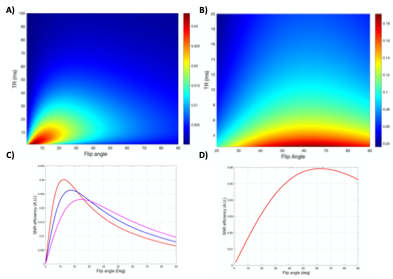
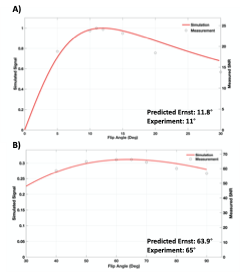
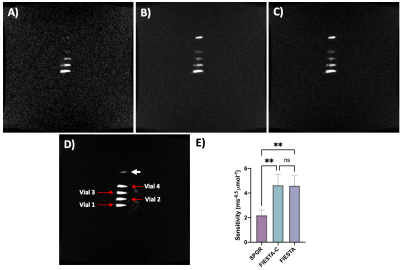
Simulation results (Figure 2) demonstrate that FAs of approximately 12° and 64° would maximize the achieved signal for SPGR and FIESTA, respectively. Furthermore, the minimum possible rBW and corresponding minimum TR and TE should be selected to maximize the SNReff. Validation of optimal FA (Figure 3) demonstrates close agreement with Bloch simulation, with the experimental Ernst angle being 11° and 65° for SPGR and FIESTA, respectively. To avoid severe distortions seen in SPGR and excessive banding in FIESTA, an rBW of 10 kHz was chosen; however, banding was ultimately unavoidable at rBW < 100 kHz. Ten-minute SPGR, FIESTA, and FIESTA-C sequences achieved sensitivities of 2.2, 4.6, and 4.6 ms-0.5μmol-1,respectively. A 2-fold (p-value < 0.01) improvement in sensitivity was seen with FIESTA and FIESTA-C compared with SPGR (Figure 4). ARC-enabled FIESTA-C (Figure 5) demonstrated a further 2-fold scan time reduction, without compromising the qualitative visualization of any of the 4 vials. No noticeable image quality degradation was seen across the range of acceleration settings. The phase-cycling technique reduced signal drop out within regions-of-interest, ultimately increasing the mean signal and compensating for the additional time requirements by implementing FIESTA-C with ARC parallel imaging.Conclusion
The FIESTA sequence variants, as expected, demonstrated 2.1-fold improvement in sensitivity relative to SPGR; however, FIESTA was prone to off-resonance banding artifacts. Results demonstrate that FIESTA-C provides SNReff comparable to FIESTA while mitigating off-resonance effects. Furthermore, FIESTA-C with ARC was able to reduce scan times 2-fold without compromising qualitative image quality. Future work aims to demonstrate cell tracking feasibility in an ex vivo canine model using this presented FIESTA-C approach.Acknowledgements
This work was supported by grants from a National Cancer Institute/National Institutes of Health (NCI/NIH) R01 CA215461, Science and Medicine Graduate Research Scholars (SciMed GRS) Fellowship, and National Cancer Institute/ National Institutes of Health (NCI/NIH) T32 CA009206 (LML).
References
1. Chapelin, F., Capitini, C. M. & Ahrens, E. T. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. J. Immunother. Cancer 6, 1–11 (2018).
2. Colotti, R. et al. Characterization of perfluorocarbon relaxation times and their influence on the optimization of fluorine-19 MRI at 3 tesla. Magn. Reson. Med. 77, 2263–2271 (2017).
3. Ahrens, E. T., Helfer, B. M., O’Hanlon, C. F. & Schirda, C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn. Reson. Med. 72, 1696–1701 (2014).
4. Darçot, E. et al. Towards Quantification of Inflammation in Atherosclerotic Plaque in the Clinic - Characterization and Optimization of Fluorine-19 MRI in Mice at 3 T. Sci. Rep. 9, 17488 (2019).
5. Rose, L. C. et al. Fluorine-19 Labeling of Stromal Vascular Fraction Cells for Clinical Imaging Applications. Stemcells Transl. Med. 2, 1472–1481 (2015).
6. Deoni, S. C. L., Ward, H. A., Peters, T. M. & Rutt, B. K. Rapid T2 estimation with phase-cycled variable nutation steady-state free precession. Magn. Reson. Med. 52, 435–439 (2004).
7. Zur, Y., Wood, M. L. & Neuringer, L. J. Motion‐insensitive, steady‐state free precession imaging. Magn. Reson. Med. 16, 444–459 (1990).
Figures