1361
STEAM-based proton observed carbon edited (POCE) 13C magnetic resonance spectroscopy without decoupling at 7T1Animal imaging and technology (CIBM), EPFL, Lausanne, Switzerland, 2Laboratory for Functional and Metabolic Imaging (LIFMET), EPFL, Lausanne, Switzerland
Synopsis
1H-[13C] NMR spectroscopy is a powerful tool to study metabolic information in human brain. At 7T, the proton-observed carbon-edited (POCE) method provides high sensitivity and improved spectral resolution. However, the application in human brain has more constraints due to high RF power deposition at 7T. In this study, we investigated the performance of the STEAM-based POCE sequence without decoupling to reduce the RF power. The phantom experiments and the Monte Carlo simulations demonstrated high-quality 1H-[13C] MR spectra and the accurate quantification of 13C-labeled glutamate and glutamine without decoupling at 7T.
Introduction
The 13C NMR spectroscopy combined with 13C-labeled substrate administration is an outstanding technique to study the metabolic pathways in human brain. The 13C label incorporation into metabolites, such as glutamate and glutamine, can be detected when using 13C-labeled glucose or acetate infusion1. The proton-observed carbon-edited (POCE) method provides high sensitivity and accuracy2. However, the spectral resolution is limited. At low to moderate magnetic field strengths, the spectral editing scheme was used to select and separate the labeling resonances of glutamate and glutamine3. The spectral resolution gets improved at 7T, so less overlapping of Glu-H4 and Gln-H4 is expected. The increasing Specific Absorption Rate (SAR) challenge at high magnetic fields limits the application of sensitivity enhancement techniques such as decoupling and Nuclear Overhauser Enhancement (NOE), especially in brain regions with the high local power deposition such as the frontal lobe. The POCE based on stimulated echo acquisition mode (STEAM) without 13C decoupling can effectively reduce the RF power deposition and achieve accurate localization. Nevertheless, the 1H-[13C] MR spectra would be further complicated and the sensitivity will decrease when omitting 13C decoupling, which may result in compromised measurement reliability. Therefore, in this study, we aim to (1) adapt and implement the selective Resonance suppression by Adiabatic Carbon Editing single-voxel STimulated Echo Acquisition Mode (RACE-STEAM)4 sequence on the 7T human scanner. (2) evaluate the quantification reliability of 13C-labeled glutamate and glutamine of non-decoupled 1H-[13C] MR spectra, and compare the quantification accuracy with Gln-C4/Glu-C4 suppression method and non-suppression method using simulations.Methods
MaterialsMR measurements were performed on a 7T human MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a dual tuned 1H-13C RF surface coil, which consists of a quadrature 1H coil (2 circular loops, diameter 9cm) and a linear 13C coil (1 circular loop, diameter 6cm)5. A 1.5L self-built cylindrical phantom containing 100mM glutamate and 25mM glutamine was used (natural abundance of 13C ~ 1.1%).
MR spectroscopy
The ultra-short TE STEAM sequence was implemented with VAPOR water suppression and interleaved Outer Volume Suppression (OVS)6. Two asymmetric narrow-transition-band adiabatic RF inversion pulses7 (consisting of the first half of 4ms HS1/2 pulse with R=0.5 and the second half of 36ms tanh/tan pulse with R=400) were applied alternatively on the 13C channel during TM. The transition bandwidth is 80Hz and the inversion bandwidth is 3.5kHz at a γB1/2π of 1.5kHz. A 38×16×20mm3 voxel near the surface of the coil (TR = 4s, TE = 7.9ms, TM = 50ms, 256 averages, bandwidth = 4kHz, voxel size = 2048) was used to acquire the spectra.
We performed two 1H-[13C] MR spectrum acquisition schemes based on RACE-STEAM by moving the 13C transmitter frequency: (1) 2-scan non-suppression scheme: 13C transmitter frequency is set away from the frequency of C2, C3, and C4 of glutamate and glutamine (60ppm) for two scans. (2) 4-scan suppression scheme: For the first two scans, 13C transmitter frequency is set to Glu-C4 (34.2ppm) to suppress the [4-13C]-Glu-H4 resonances. For the third and fourth scans: 13C transmitter frequency is set to Gln-C4 (31.7ppm) to suppress the [4-13C]-Gln-H4 resonances.
Data analysis and simulations
FID-A was used to process the phantom spectra. The simulation of 1H-[13C] MR spectra and the basis sets were realized in MATLAB R2020a (The MathWorks Inc., Natick, MA, USA). The 1H and 13C chemical shifts and J-coupling constants were taken from the literature8,9,10. The time-course data was taken from Gruetter et al.11. The non-decoupled 1H-[13C] MR spectra at 7T acquired by two schemes were simulated with a linewidth of 12Hz. Metabolite quantification was performed in LCModel (Stephen Provencher Inc. Oakville, ON, Canada). The estimated error (EE = (Cmeasured-Cground truth)/Cground truth×100%) and the Cramér-Rao Lower Bound (CRLB) were evaluated by Monte-Carlo simulations (100 measurements/condition).
Results
At 7T, the satellites of Gln-H4 overlap less on Glu-H4 (figure 2(a)). For Glu-H2 (3.74ppm) and Gln-H2 (3.75ppm), Glu-H3 (2.03-2.12ppm) and Gln-H3 (2.10-2.12ppm), their satellite peaks are overlapped. The excellent suppression of Glu-H4 and Gln-H4 was validated and shown in Figures 2(b1) and (b2) by using the 4-scan suppression scheme in phantom experiments. Simulated 1H-[13C] MR spectra after [1-13C] glucose infusion by using 2-scan and 4-scan schemes provide comparable accuracy. The Monte Carlo simulations indicate the quantification of C2, C3, and C4 of glutamate and glutamine is reliable after 10 minutes as shown in figure 3. The EEs and CRLBs of the metabolite concentrations decrease over time. The CRLB of Gln-C4 is relatively larger due to the lower concentration for both 2-scan and 4-scan methods, decreasing from 13% to 3% over time, while others are only 1%-2%.Discussion and Conclusion
The RACE-STEAM sequence without 13C decoupling can effectively minimize the RF power deposition and chemical shift displacement at 7T. We have illustrated that the quantification accuracy of Glu-C4, Gln-C4, Glx-C3, and Glx-C2 by non-decoupled 1H-[13C] MR spectra with the non-suppression scheme and the suppression scheme are comparable. Separation of non-decoupled [4-13C]-Glu-H4 and [4-13C]-Gln-H4 without suppression is considered sufficient for their reliable quantifications at 7T. We conclude that RACE-STEAM without decoupling could offer reliable 13C labeling measurement at 7T and allow its potential application in human brain regions with the high SAR challenge.Acknowledgements
This work was supported by the Swiss National Science Foundation (grants n° 320030_189064). We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence funded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole Polytechnique Fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).
References
1. Henry, P.G., Adriany, G., Deelchand, D., Gruetter, R., Marjanska, M., Öz, G., Seaquist, E.R., Shestov, A. and Uğurbil, K., 2006. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magnetic resonance imaging, 24(4), pp.527-539.
2. Chen, H., De Feyter, H.M., Brown, P.B., Rothman, D.L., Cai, S. and de Graaf, R.A., 2017. Comparison of direct 13C and indirect 1H-[13C] MR detection methods for the study of dynamic metabolic turnover in the human brain. Journal of Magnetic Resonance, 283, pp.33-44.
3. De Feyter, H.M., Herzog, R.I., Steensma, B.R., Klomp, D.W., Brown, P.B., Mason, G.F., Rothman, D.L. and de Graaf, R.A., 2018. Selective proton‐observed, carbon‐edited (selPOCE) MRS method for measurement of glutamate and glutamine 13C‐labeling in the human frontal cortex. Magnetic resonance in medicine, 80(1), pp.11-20.
4. Xin, L., Frenkel, H., Mlynárik, V., Morgenthaler, F.D. and Gruetter, R., 2009. Selective resonance suppression 1H‐[13C] NMR spectroscopy with asymmetric adiabatic RF pulses. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 61(2), pp.260-266.
5. Roig, E.S., Magill, A.W., Donati, G., Meyerspeer, M., Xin, L., Ipek, O. and Gruetter, R., 2015. A double‐quadrature radiofrequency coil design for proton‐decoupled carbon‐13 magnetic resonance spectroscopy in humans at 7T. Magnetic resonance in medicine, 73(2), pp.894-900.
6. Tkáć, I. and Gruetter, R., 2005. Methodology of 1 H NMR spectroscopy of the human brain at very high magnetic fields. Applied magnetic resonance, 29(1), pp.139-157.
7. Hwang, T.L., Van Zijl, P.C. and Garwood, M., 1999. Asymmetric adiabatic pulses for NH selection.
8. Govindaraju, V., Young, K. and Maudsley, A.A., 2000. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo, 13(3), pp.129-153.
9. Henry, P.G., Öz, G., Provencher, S. and Gruetter, R., 2003. Toward dynamic isotopomer analysis in the rat brain in vivo: automatic quantitation of 13C NMR spectra using LCModel. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo, 16(6‐7), pp.400-412.
10. Deelchand, D.K., Uğurbil, K. and Henry, P.G., 2006. Investigating brain metabolism at high fields using localized 13C NMR spectroscopy without 1H decoupling. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 55(2), pp.279-286.
11. Gruetter, R., Seaquist, E.R. and Ugurbil, K., 2001. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. American Journal of Physiology-Endocrinology And Metabolism, 281(1), pp.E100-E112.
Figures
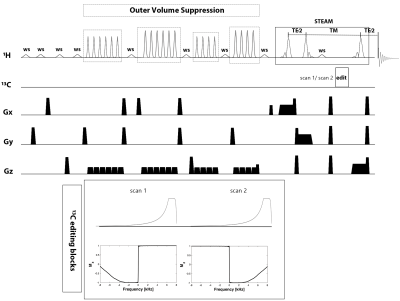
Figure 1. Diagram of RACE-STEAM sequence. The pulse train of VAPOR water suppression (ws) is interleaved with four blocks of Outer Volume Suppression (OVS) modules. Two asymmetric narrow-transition-band adiabatic RF inversion pulses are applied alternatively during the TM. 1H-[13C] MR spectra are given by the subtraction of the two scans.
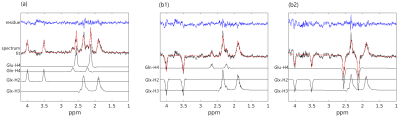
Figure 2. Experimentally acquired 1H-[13C] MR spectra via RACE-STEAM from the phantom and LCModel fits. (a) 13C transmitter frequency offset was set to 60.0±0.2ppm; (b1) 13C transmitter frequency offset was set to 34.5±0.2ppm to suppress the satellites of Glu-H4; (b2) 13C transmitter frequency offset was set to 31.0±0.2ppm to suppress the satellites of Gln-H4.
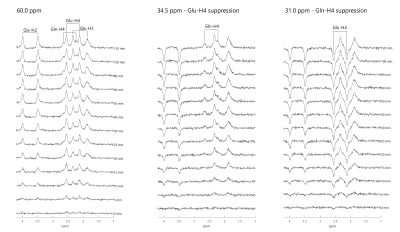
Figure 3. Simulated 1H-[13C] MR spectra by 2-scan scheme (13C frequency offset at 60ppm) and 4-scan scheme (13C frequency offset at 34.5ppm and 31ppm) over time after [1-13C] glucose infusion, with a linewidth of 12Hz. The noise with a root mean square of 1/20 of [4-13C]-Gln-H4 resonance at 120 min was added to all simulated spectra.
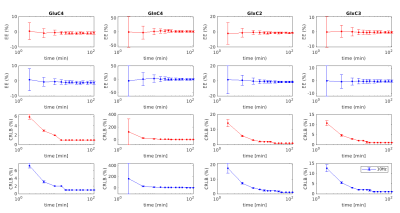
Figure 4. Estimated errors (EE) and CRLBs of the 2-scan scheme (red) and the 4-scan scheme (blue) were compared at different time points. The EEs, CRLBs, and standard deviations were computed from 100 simulated spectra with the same SNR for each time point.