1360
An old tracer learns new tricks: Imaging cerebral glucose uptake with deuterated 2-deoxy-d-glucose-2,2-d211 Department of Physical Therapy and Rehabilitation Science, University of California, San Francisco, San Francisco, CA, United States, 2Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 3UC Berkeley-UCSF Bioengineering Program, University of California, San Francisco, San Francisco, CA, United States
Synopsis
2-deoxy-d-glucose (2-DG) is a glucose analog widely used in FDG-PET imaging as a biological tracer of glucose uptake. This study innovatively redirects its application in 2H NMR by using a deuterated version of 2-DG (2-DG-d2) and explores its imaging potential in mapping glucose uptake without ionizing radiation. A workflow is described here regarding multi-band RF pulse design and bSSFP sequence optimization, which enables a rapid 2H imaging with high sensitivity. Both in vitro and in vivo imaging results have validated the pulse sequence’s specificity and sensitivity of detecting 2-DG-d2 with high spatial resolution, inspiring its further implementation in the future.
Introduction
Deuterium (2H) Nuclear Magnetic Resonance (NMR) was first proposed in the 1960’s1. A marginal NMR technique for decades since2, Deuterium Metabolic Spectroscopy (DMS) and Imaging (DMI) are being recently recognized as a powerful method in inferring and mapping metabolic activities3. Along with the resurgence of DMI/S, new deuterated substrates are being investigated4.Here, we propose a novel utilization of DMI in imaging a glucose analog, 2-deoxy-d-glucose-2,2-d2 (2-DG-d2) (Fig.1A inset). After being taken up through glucose transporters, 2-DG is trapped in the cell post phosphorylation and cannot undergo glycolysis, like 18F-FDG-PET (Fig.1B). This trapping feature discharges 2-DG-d2 imaging from the need for high-resolution spectral encoding, enabling rapid 2H imaging sequences with high spatiotemporal resolution and sensitivity. Our results show that by leveraging a multi-band spectrally-selective RF pulse and a balanced Steady-State Free Precession (bSSFP) sequence at 14.1T, we can map the distribution of 2-DG-d2 in the healthy mouse brain 140min after IP injection with high spatial resolution (1.25x1.25x2.5mm3) and SNR in under 20 minutes. Such an outcome suggests that 2-DG-d2 imaging could probe glucose uptake, similarly to 18F-FDG-PET, but without the need for a cyclotron and without ionizing radiation.
Methods
18.8T NMR:2-DG-d2 was custom-synthesized by Sigma-Aldrich® ISOTEC. To characterize 2-DG-d2, 5mm NMR tubes filled with 30mM 2-DG-d2 in 1xPBS underwent 1D spectroscopy on an 18.8T Bruker® system, using: hard pulse (100𝜇s, FA=90o), TR=4s, NEX=8, readout=1.31s, SW=12.5kHz.
14.1T Phantom Study:
A multi-band spectrally-selective RF pulse for 2-DG-d2 was generated using the CVX pulse design toolbox as described before5. To evaluate the pulse’s specificity to 2-DG-d2, a 100mM 2-DG-d2 phantom was tested by a 2H 2D-CSI sequence at a 14.1Tesla Agilent® system. A 16mm surface coil from Doty Scientific® was used for both transmission and reception. The scan parameters for 2D-CSI: TR/TE=1000ms/4ms, FA=90o, readout=51ms, SW=10kHz, matrix size=8x8, voxel size=2.5x2.5mm2, NEX=8, total scan time=8min32s.
To improve the detection sensitivity of 2-DG-d2, a bSSFP 3D-GRE sequence was optimized via Bloch simulation and tested on phantoms containing 10mM or 100mM 2-DG-d2. The scan parameters were: TR/TE=9ms/4.5ms, FA=60o, readout=1.99ms, SW=8kHz, matrix size=16x16x8, voxel size=1.25x1.25x2.5mm3, NEX=600, total scan time=11min40s.
14.1T in vivo Study:
With using the same hardware as the phantom study, two different 2H sequences were tested in healthy mice. To confirm the spectral selectivity of the multiband RF pulse, 2D-CSI was acquired in a healthy mouse without 2-DG-d2 injection, using parameters: TR/TE=1000ms/4ms, FA=90o, readout=51ms, SW=10kHz, matrix size=8x8, voxel size =2.5x2.5mm2, NEX=20, total scan time = 21m20s. Furthermore, the optimized bSSFP 3D-GRE was run to acquire 2H imaging post 2-DG-d2 injection (3g/kg BW, IP), using: TR/TE=9ms/4.5ms, FA=60o, readout=1.99ms, SW=8kHz, voxel =1.25x1.25x2.5mm3, NEX=1000, scan time=19min21s.
Results & Discussion
The 18.8T spectrum of 30mM 2-DG-d2 shows two doublets centered at ~2.9ppm upfield of HDO (Fig.1A). All four 2-DG-d2 peaks span ~0.8ppm, which corresponds to a bandwidth of ~74Hz at 14.1T.Under the design constraints, the shortest pulse solution resolved by CVX toolbox has a duration of 6ms and a peak amplitude of 0.47G (Fig.2A), providing greater HDO stopband suppression than a standard minimum-phase pulse. The spectral response of the selective pulse met our design criteria in fully exciting the bandwidth of 2-DG-d2 (min|Mxy|>0.95) while suppressing HDO (max|Mxy|<0.01) (Fig.2B).
Relaxation measurement study at 14.1T gave an estimated T1/T2 of HDO=320ms/78ms, and T1/T2 of 2-DG-d2=47ms/47ms (Fig.2C). Given the near unity T2/T1 ratio for 2-DG-d2, a bSSFP acquisition is an appealing pulse sequence because of its significant SNR improvement over spoiled-GRE sequences. By utilizing this relaxation information, a Bloch simulation of the bSSFP signal response using the spectrally-selective pulse (TR/TE=9ms/4.5ms) shows that at steady state (>30 iterations of TR) an optimal FA=60o resulted in the highest average signal intensity from 2-DG-d2 bandwidth, providing >500-fold suppression of HDO signal (Fig.2D).
The 2H 2D-CSI results from both phantom and in vivo studies have demonstrated the robust specificity of the selective pulse towards 2-DG-d2 (Fig.3B & Fig.4B), where HDO signal was below the noise floor when compared to a non-selective hard pulse. Regarding sensitivity, bSSFP 3D-GRE with selective pulse was capable of detecting signal from a 10mM 2-DG-d2 phantom within ~12min (Fig.3C). At 140min post IP injection of 2-DG-d2, the same bSSFP 3D-GRE sequence was able to detect the distribution of intracranial 2-DG-d2 by a ~20min scan at high spatial resolution (Fig.5B). On the contrary, when applying the same scan to a control animal without receiving 2-DG-d2 injection, the 2H imaging signal was below the noise floor (Fig.5D).
Conclusions
In this methodology study, the NMR attributes of 2-DG-d2 have been quantified and utilized for a bSSFP sequence optimization that provides a significant increase in SNR over 2D-CSI, enabling high-resolution volumetric imaging (1.25x1.25x2.5mm3). A multi-band spectrally-selective RF pulse was generated from an open-source toolbox, and proved to be robust and selective to 2-DG-d2 in both in vitro and in vivo conditions. The synergy of bSSFP and spectrally-selective pulse has provided an encouraging sensitivity of mapping physiological level of 2-DG-d2 inside the brain. A further optimization in k-space sampling method can shorten the scan time and improve SNR, which will lead to a more efficient way of imaging and quantifying glucose uptake activity.Acknowledgements
This work was supported by research grants: R01AG064170, R01NS102156, and R61NS115132. Sincere appreciation to Dr. Tan from Sigma-Aldrich® ISOTEC for assistance in synthesizing the 2-deoxy-d-glucose-2,2-d2 samples.
References
1. Diehl P, Leipert T (1964) Helv Chim Acta 47: 545
2. Martin ML, Martin GJ. Deuterium NMR in the study of Site-Specific Natural Isotope Fractionation (SNIF-NMR). (1991) in NMR (Series) v. 23 (Diehl P, Fluck E, Günther E, Kosfeld R, and Seelig J, eds), pp. 3-61, Springer-Verlag Berlin Heidelberg
3. Henk M. De Feyter, Robin A. de Graaf, Deuterium metabolic imaging – Back to the future, Journal of Magnetic Resonance, Volume 326, 2021, 106932, ISSN 1090-7807
4. Polvoy I, Qin H, Flavell RR, et al. Deuterium Metabolic Imaging-Rediscovery of a Spectroscopic Tool. Metabolites. 2021;11(9):570. Published 2021 Aug 25. doi:10.3390/metabo11090570
5. Shang H, Larson PEZ, Kerr A, et al. Multiband RF pulses with improved performance via convex optimization. J Magn Reson. 2016;262:81-90. doi:10.1016/j.jmr.2015.11.010
Figures
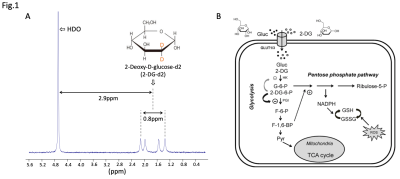
(A) Spectrum of 30mM 2-DG-d2 (dissolved in 1xPBS) acquired at 18.8Tesla, showing the two doublets of 2-DG-d2 located ~2.9ppm away from HDO. (B) The metabolic fate of 2-DG, a glucose analog. 2DG is trapped inside cell post phosphorylation and cannot undergo downstream glycolysis. The absence of metabolic products provides a sparse spectral profile for in vivo studies, eliminating the need for high-resolution spectral encoding.
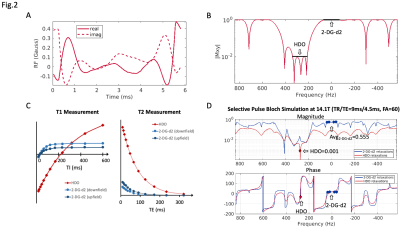
(A) A multi-band spectrally-selective RF pulse for 2-DG-d2 was generated from CVX5. The resolved pulse solution has a duration of 6ms and a peak amplitude of 0.47G. (B) Theoretical spectral response. (C) T1/ T2 of a 1M 2-DG-d2 phantom at 14.1T (HDO=320ms/78ms, 2-DG-d2 = 47ms/47ms). (D) A Bloch simulation of the bSSFP signal response using the spectrally-selective pulse, TR/TE=9ms/4.5ms. An optimal FA=60o secures the highest average signal intensity from 2-DG-d2 bandwidth (>500-fold higher than HDO). Phase is consistent across the 2-DG-d2 bandwidth.

(A) 1H image and 2H 2D-CSI overlay of two phantoms containing 10mM (left) and 100mM (right) 2-DG-d2. (B) 2H spectra from the red box on the left, acquired using a non-selective hard pulse (red) and a spectrally-selective pulse (blue), where only 2-DG-d2 peak was detected (C) bSSFP 3D-GRE with spectrally-selective pulse provides enough sensitivity to detect 2-DG-d2 signal from the 10mM phantom.
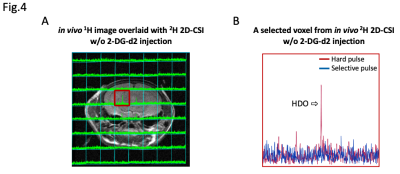
(A) 1H image of a healthy mouse brain that did not receive a 2-DG-d2 injection, overlaid with a grid of 2H 2D-CSI (TR/TE=1000ms/4ms, FA=90o, readout=51ms, SW=10kHz, matrix size=8x8, voxel size =2.5x2.5mm3, NEX=20, total scan time = 21m20s). (B) 2H spectra from the red box on the left, acquired using a non-selective hard pulse (red) and a spectrally-selective pulse (blue). HDO peak can only be detected from hard pulse result; HDO signal from selective pulse result is under noise floor, validating the spectral selectivity of the multiband 2-DG-d2 RF pulse in vivo.
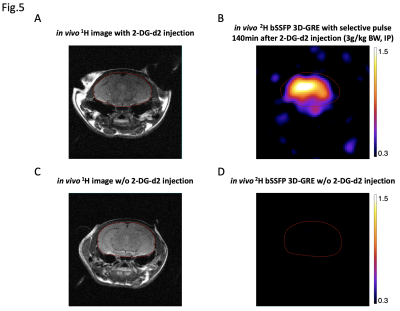
(A) 1H image of a healthy mouse brain and (B) the corresponding heatmap acquired 140min after 2-DG-d2 injection using a 2H bSSFP 3D-GRE with spectrally-selective pulse. 4x zero-filling and 10dB Gaussian filtering were applied for post-processing. (C) & (D) Same 1H and 2H scans from a healthy mouse without receiving 2-DG-d2 injection, where 2-DG-d2 signal is below the noise floor. A significant SNR difference in ROIbrain between 2-DG-d2 and control animals confirms the pulse sequence’s specificity to 2-DG-d2 in vivo.