1351
Double Quantum Filtered 2H Measurements in Human Subjects Following Deuterium Oxide Loading
Robin Damion1,2,3, Daniel Cocking3,4, Hester Franks1, Daniel Wilkinson5,6, Matthew Brook2,6,7, Dorothee Auer1,2,3, and Richard Bowtell2,3,4
1School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2NIHR Nottingham Biomedical Research Centre/Nottingham Clinical Research Facilities, University of Nottingham, Nottingham, United Kingdom, 3Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 4School of Physics & Astronomy, University of Nottingham, Nottingham, United Kingdom, 5Division of Medical Sciences and Graduate Entry Medicine, University of Nottingham, Nottingham, United Kingdom, 6MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Nottingham, Nottingham, United Kingdom, 7School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
1School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2NIHR Nottingham Biomedical Research Centre/Nottingham Clinical Research Facilities, University of Nottingham, Nottingham, United Kingdom, 3Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 4School of Physics & Astronomy, University of Nottingham, Nottingham, United Kingdom, 5Division of Medical Sciences and Graduate Entry Medicine, University of Nottingham, Nottingham, United Kingdom, 6MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Nottingham, Nottingham, United Kingdom, 7School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
Synopsis
Double-quantum filtered (DQF) deuterium spectra were obtained on a 3T scanner from lower leg and forearm muscles of volunteers whose deuterium abundance was increased by approximately 100 times through ingestion of deuterium oxide. Quadrupolar splitting frequencies of approximately 20 – 40 Hz were measured throughout the various muscle groups of the lower leg, with some regions showing little or no splitting. Although the DQF sequence considerably reduces the signal intensity, it has the advantage that it removes any isotropic signal component and therefore reveals an anisotropic component that might have been obscured.
Introduction
Ordered biological tissues and substructures can be studied via deuterium (2H) magnetic resonance because its strong quadrupolar interaction produces a frequency doublet which is sensitive to the effect of ordering on the time-averaged direction of the local electric field gradient with respect to the magnetic field1,2. The low natural abundance of deuterium (0.015%) means that performing such measurements in vivo without deuterium enrichment can be challenging, but achievable at high field3. However, tissues typically contain multiple water compartments and spectra can be complicated by the superposition of ordered (anisotropic) and disordered (isotropic) signal components. One method of eliminating the signal from isotropic components is by employing a double quantum filter (DQF)4,5, but this also significantly reduces the detectable signal. Therefore, taking advantage of a parallel study on immune cell proteomics, in which healthy human subjects ingested deuterium oxide (D2O) to produce ~1.5% 2H enrichment over six weeks, we measured 2H DQF spectra from muscle in the presence of the enhanced signal.Methods
Deuterium spectroscopy and chemical shift imaging (CSI) were performed on lower legs and forearms in four human volunteers, using in-house-built 2H coils resonating at 19.6 MHz, interfaced to a Philips Achieva 3T imaging system. A 16cm-diameter saddle coil was used for the lower leg, and a 15cm-diameter Helmholtz coil was used for the forearm. All measurements were performed with the limb parallel to the magnetic field direction.Deuterium spectra were acquired from a 2 cm axial slice of the lower leg or forearm, by using hard pulses in combination with outer-volume saturation. DQF spectra were created by using the sequence in Figure 1, which produces an anti-phase spectrum whereby the quadrupolar doublet peaks acquire a relative phase of 180$$$^{\circ}$$$ (see Figure 2). This relative phase means that the signal vanishes for any components if their splitting frequency is small. DQF spectra are shown in subsequent figures, phased to produce a symmetric line-shape (labelled as dispersion spectra in anti-phase in Figure 2).
DQF spectra were acquired for various values of the creation time, $$$\tau$$$, (see Figure 1) and TR/TE=1000/0.58 ms, sampling bandwidth 3000 Hz, 1024 samples. The number of averages varied, depending on available signal, between 28 and 128. FIDs were processed using Matlab scripts and spectra were fitted to two independent Lorentzian line shapes.
Deuterium CSI data were also acquired from a single 2 cm axial slice, again using outer-volume suppression. CSI data were acquired for the usual pulse-acquire sequence, and using the anti-phase DQF sequence with $$$\tau=6.5$$$ ms. The in-plane resolution was $$$10\times 10$$$ mm2, TR/TE=378/3.9 ms, 256 samples were acquired at a bandwidth of 750 Hz. For the DQF CSI, 16 averages were acquired, whereas only 4 were needed for the pulse-acquire CSI.
Results
Figure 3 shows 2H CSI data overlaid on 1H gradient-echo images of the lower leg, for both the DQF CSI and the pulse-acquire CSI sequences. Individual DQF and pulse-acquire spectra from three voxels are also shown in more detail. The characteristic ‘sinc-like’ shape (Figure 2) can be seen in the DQF spectra, while the in-phase doublet pattern is seen in some voxels in the pulse-acquire data.Figure 4 shows examples of four, phase-corrected, DQF spectral datasets (three lower leg, one forearm) acquired from 2 cm axial slices, acquired with a range of $$$\tau$$$-values. Each spectrum was fitted to two independent Lorentzians (as illustrated in Figure 2) from which the mean amplitude was plotted as a function of $$$\tau$$$. Examples of these DQF ‘build-up’ plots are shown in Figure 5 (two forearms, two lower legs).
Discussion
In the ideal case of perfect flip angles and the signal (central frequency) being exactly on-resonance, the DQF build-up curves, shown in Figures 4 and 5, follow the damped sinusoidal form $$$A\sin ( 2\pi\nu_q\tau ) \exp ( -2\tau/T_2 )$$$, where $$$\nu_q$$$ is the reduced quadrupolar splitting frequency4. Although the curves in Figure 5 are fitted to this form, it is clear that they do not perfectly model the data, most likely due to flip-angle errors, small resonance offsets, and spread of quadrupolar frequencies4. Fits to eight DQF datasets produced a mean and standard deviation of $$$\nu_q=39.7\pm 11.5$$$ Hz. However, in all curves, a global maximum is seen at approximately $$$2\tau\approx 15$$$ ms, which corresponds approximately to $$$\nu_q\approx 33$$$ Hz. As a comparison, mean values obtained by fitting all DQF spectra in each dataset produced $$$\nu_q=29.5\pm 3.7$$$ Hz, which is in closer agreement with values similarly measured in the lower leg by Gursan et al.3From the spectra in Figure 3, we also observe that the splitting frequencies tend to be larger in the tibialis anterior, which is primarily believed to result from the muscle group being more closely aligned with the magnetic field direction.
Conclusions
We have shown that 2H DQF measurements can be made on a 3T scanner in skeletal muscle in human subjects and that although the resulting spectra are reduced in amplitude, the elimination of isotropic water components simplifies the line shapes and separates out signals from ordered compartments. Further work is required to understand the precise origin of the quadrupolar splitting in muscle in relation to its structure and the existence of bound water compartments6.Acknowledgements
This research was funded by the NIHR Nottingham Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. DJC’s Ph.D. studies are funded by the Precision Imaging Beacon at the University of Nottingham. The pilot study into immune cell responses in cancer is supported by Cancer Research UK [A29820].References
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Quarterly Reviews of Biophysics. 1977; 10(3): 353 – 418.
- Eliav U, Navon G. Multiple Quantum MRS. eMagRes. 2016; 5(1). DOI: 10.1002/9780470034590.emrstm1448
- Gursan A, Froeling M, Hendriks AD, Welting D, Kentgens APM, Klomp DWJ, Prompers JJ. Residual quadrupolar couplings observed in 7 Tesla deuterium MR spectra of skeletal muscle. Magnetic Resonance in Medicine. 2021; 00: 1 – 9. DOI: 10.1002/mrm.29053
- Sharf Y, Eliav U, Shinar H, Navon G. Detection of anisotropy in cartilage using 2H double-quantum-filtered NMR spectroscopy. Journal of Magnetic Resonance. 1995; B107: 60 – 67.
- Perea W, Cannella M, Yang J, Vega, AJ, Polenova T, Marcolongo M. 2H double quantum filtered (DQF) NMR spectroscopy of the nucleus pulposus tissues of the intervertebral disc. Magnetic Resonance in Medicine. 2007; 57: 990 – 999.
- Eliav U, Wehli FW, Navon G. New insight into the organisation of myelin water using deuterium NMR. Magnetic Resonance in Medicine. 2020; 84(2): 535 – 541.
Figures
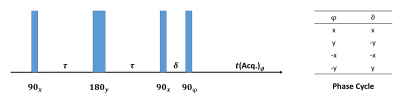
Figure 1. The anti-phase DQF sequence4. The time period $$$\delta$$$ was fixed at 0.1 ms. The $$$\tau$$$ values used in experiments were typically of the order of 1 – 20 ms, and can be seen in the graphs of Figures 4 and 5. The phase cycle shown suppresses zero-quantum and single-quantum coherences.
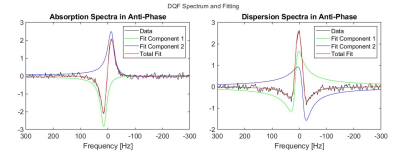
Figure 2. DQF spectrum from an axial 2 cm slice of the lower leg, acquired with $$$\tau=6.5$$$ ms. The plots show the fitting of the two independent Lorentzians to the anti-phase DQF doublet. The fitting produced a value for the splitting of $$$\nu_q=27.7$$$ Hz, and mean $$$ T_2^{\ast}=11.3$$$ ms.
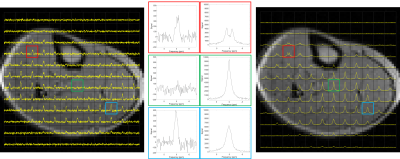
Figure 3. Deuterium DQF CSI (left) and pulse-acquire CSI (right) data obtained from a 2 cm axial slice of the lower leg of the same volunteer with 1cm in-plane resolution. In the centre are spectra from three selected voxels so that a direct comparison can be made of the DQF (left) and pulse-acquire (right) spectra from different muscle regions.
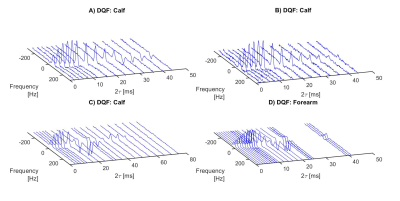
Figure 4. Deuterium DQF spectra acquired from a 2 cm slice of the lower leg (of different volunteers) and forearm with different values of the creation time $$$\tau$$$.
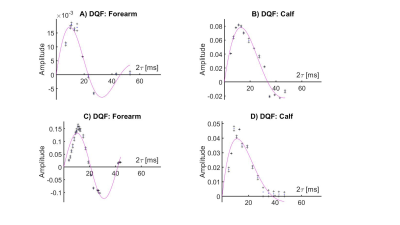
Figure 5. DQF amplitudes obtained by fitting anti-phase line shapes to spectra obtained from the lower leg and forearm (of different volunteers) as a function of the creation time $$$\tau$$$. Continuous magenta lines show fits to the DQF amplitude variation with $$$\tau$$$ of the form $$$A\sin ( 2\pi\nu_q\tau) \exp ( -2\tau/T_2)$$$. The amplitude term varies across datasets because of the use of different RF coils and the different levels of D2O loading in different volunteers.
DOI: https://doi.org/10.58530/2022/1351