1350
Optimal acquisition settings for simultaneous diffusion kurtosis, free water fraction and T2 estimation1imec-Vision Lab, Dept. of Physics, University of Antwerp, Antwerp, Belgium, 2icometrix, Leuven, Belgium, 3µNEURO Research Centre of Excellence, University of Antwerp, Antwerp, Belgium
Synopsis
Fitting the diffusion kurtosis imaging free water elimination model (DKI-FWE) to diffusion MRI data represents an ill-conditioned problem. Fortunately, the conditioning of the model fitting can be improved by explicitly modeling the T2 relaxation dependency of the signal. As a benefit, diffusion and kurtosis metrics robust to partial volume effects can be estimated with conventional techniques. In this work, we use Cramér-Rao lower bound (CRLB) theory to identify optimal acquisition settings that maximize the precision of the model parameter estimates.
Introduction
The two-compartment diffusion kurtosis imaging free water elimination model (DKI-FWE)1 allows to mitigate the bias induced by partial volume effects on tissue-related diffusion parameters. The incorporation of the compartmental $$$T_2$$$ relaxation times helps stabilize the FWE model fitting problem2. Therefore, accurate and precise parameter estimates can be obtained by using standard nonlinear least squares (NLLS) estimation techniques. However, DKI is a lengthy imaging protocol, implying that the choice of the data acquisition protocol plays a crucial role to achieve reliable quantifications of the tissue properties in competitive acquisition times. In this work, we optimize the diffusion weighting strengths ($$$b$$$-values) and echo times (TEs) in terms of the Cramér-Rao lower bound (CRLB) variances of the model parameters and derived metrics3.Methods
DKI-FWE with explicit $$$T_2$$$-dependency (in short $$$T_2$$$-DKI-FWE) models the $$$T_2$$$ and diffusion weighted signal as: $$S = S_{00} \left( (1-f) \mathrm{exp}\left(-\mathrm{TE}/T_2^{\mathrm{tissue}}\right) S_{\mathrm{tissue}}(b, \mathbf{g};\boldsymbol{\theta}_{\mathrm{DKI}}) + f \mathrm{exp}\left({-\mathrm{TE}/T_2^{\mathrm{fw}}}\right) \mathrm{exp}(-bd) \right),$$ with $$$S_{00}$$$ the non-diffusion weighted signal at TE = 0 ms, $$$f$$$ the TE-independent free water signal fraction, $$$\mathbf{g}$$$ the diffusion weighting direction, $$$\boldsymbol{\theta}_{\mathrm{DKI}}$$$ the diffusion and kurtosis tensor parameter vector and $$$d$$$ the diffusivity of free water fixed to 3 μm2/ms. While $$$T_2^{\mathrm{tissue}}$$$ is estimated as an unknown model parameter, $$$T_2^{\mathrm{fw}}$$$ is fixed to a constant value of 1573 ms derived from literature4.In this work, the acquisition schemes resulting from two different CRLB-based optimality criteria ($$$O_1$$$ and $$$O_2$$$) are compared with each other and with a reference scheme ($$$R$$$) consisting of four fixed $$$b$$$-values (0, 0.5, 1 and 2 ms/μm2) acquired at two different TEs (63 and 120 ms). The optimized scheme $$$O_1$$$, previously suggested by the authors5, was obtained by minimizing the sum of the CRLB variances of $$$\boldsymbol{\theta}_{\mathrm{DKI}}$$$, $$$f$$$ and the weighted CRLB variance of $$$T_2^{\mathrm{tissue}}$$$. In the current work, we assess an additional optimized protocol resulting from the minimization of the sum of the weighted CRLB variances of the fractional anisotropy (FA), mean diffusivity (MD), the linear part of the mean kurtosis ($$$\overline{K}_{app}^{*}$$$), $$$f$$$ and $$$T_2^{\mathrm{tissue}}$$$. In line with our previous work5, 120 uniformly distributed gradient directions were kept fixed during the optimization while $$$b$$$-values and TEs were free to vary in the range 0-2 ms/μm2 and 63-121 ms, respectively. The optimality criteria were averaged over 500 white matter voxels with a certain degree of free water contamination ($$$0.05\leq f \leq 0.3$$$) and extracted from the $$$T_2$$$-DKI-FWE model fits of a real dataset. The weights applied in the optimization procedure were set equal to the inverse of the ground truth value of the parameter or metric of interest. The performance of all schemes was assessed both in terms of the maximum attainable precision (i.e. the CRLB) and the sample variances of relevant metrics (FA, MD, $$$\overline{K}_{app}^{*}$$$, $$$f$$$ and $$$T_2^{\mathrm{tissue}}$$$). The sample variances were evaluated in a single-voxel Monte Carlo simulation framework and an unconstrained NLLS fitting routine was used to prevent the introduction of any source of bias due to the position of constraints. In all experiments, Gaussian data distribution was assumed and the signal-to-noise ratio (SNR) was defined on the non-diffusion weighted signal of a non-free water contaminated voxel ($$$f$$$ = 0) at the lowest TE (i.e. 63 ms).
Results
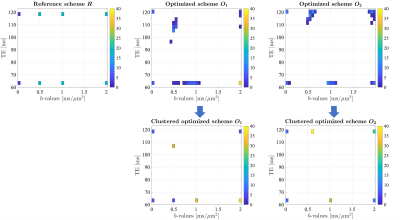
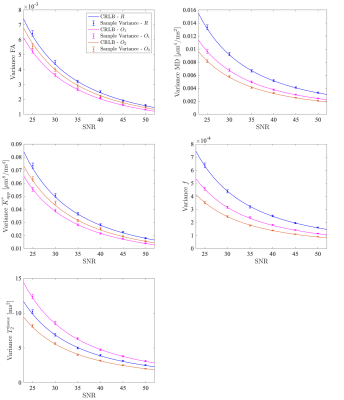
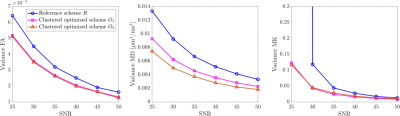
Figure 1 shows the distribution of the $$$b$$$-values and TEs for each acquisition scheme and a clustered version of the optimized schemes $$$O_1$$$ and $$$O_2$$$ is proposed for practical reasons. In Figure 2, the CRLB variances and the sample variances assessed in single-voxel experiments are compared for different metrics and for each scheme as a function of the SNR. Finally, in Figure 3, the sample variances of the FA, MD and mean kurtosis (MK) estimates are reported for the reference scheme $$$R$$$ and the clustered versions of $$$O_1$$$ and $$$O_2$$$.Discussion
The good agreement between theoretical bounds and sample variances (Fig. 2) suggests that minimizing the CRLB variances of the parameters of interest is an appropriate criterion for optimal design. Our results highlight that, depending on the parameters included in the cost function and the weighting strategy, the precision of a specific set of metrics can be favored. As a proof of concept, we compare the schemes resulting from two different optimality criteria, $$$O_1$$$ and $$$O_2$$$. Both schemes outperform the conventional acquisition protocol $$$R$$$ in terms of the precision of FA, MD, $$$\overline{K}_{app}^{*}$$$ and $$$f$$$ in a range of medium/high SNR values. When comparing $$$O_1$$$ and $$$O_2$$$, it is clear that $$$O_2$$$ leads to a gain in precision for MD, $$$f$$$ and $$$T_2^{\mathrm{tissue}}$$$ while more precise FA and $$$\overline{K}_{app}^{*}$$$ estimates can still be achieved with $$$O_1$$$. Finally, clustering the optimal settings in fixed $$$b$$$-TE combinations does not affect the precision of the diffusion derived metrics (Fig. 3) and allows to set up a more practical acquisition.Conclusion
This study shows that precise $$$T_2$$$-DKI-FWE parameter estimates can be achieved in reasonable acquisition times (< 10 minutes) by using CRLB-optimized settings. A validation of these findings on in vivo datasets will be subject of future work.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 764513. The work was also supported by the Research Foundation Flanders (FWO Belgium) through project funding (G084217N and 12M3119N).References
1. Collier Q, Veraart J, Jeurissen B, Vanhevel F, Pullens P, Parizel PM, den Dekker AJ, Sijbers J. Diffusion kurtosis imaging with free water elimination: A bayesian estimation approach. Magnetic Resonance in Medicine. 2018;80(2):802-13.
2. Collier Q, Veraart J, den Dekker AJ, Vanhevel F, Parizel PM, Sijbers J. Solving the free water elimination estimation problem by incorporating T2 relaxation properties. In Proceedings of 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA 2017 (p. 1783).
3. Poot DHJ, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE Transactions on Medical Imaging. 2010;29(3):819-29.
4. Qin Q. A simple approach for three‐dimensional mapping of baseline cerebrospinal fluid volume fraction. Magnetic Resonance in Medicine. 2011;65(2):385-91.
5. Anania V, Jeurissen B, Morez J, Buikema AE, Billiet T, Sijbers J, den Dekker AJ. Optimal experimental design for the T2-weighted diffusion kurtosis imaging free water elimination model. Book of Abstracts ESMRMB 2021 Online 38th Annual Scientific Meeting 7–9 October 2021. Magn Reson Mater Phy 34, 1-204 (2021), pp. 54-55.
Figures