1340
Open-Source Myocardial T1 mapping accelerated with SMS: combining an auto-calibrated blip-bSSFP readout with VERSE-MB pulses
Andreia S Gaspar1, Nuno A Silva2, and Rita G Nunes1
1Institute for Systems and Robotics - Lisboa and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisboa, Portugal, 2Hospital da Luz Learning Health, Luz Saúde, Lisbon, Portugal
1Institute for Systems and Robotics - Lisboa and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisboa, Portugal, 2Hospital da Luz Learning Health, Luz Saúde, Lisbon, Portugal
Synopsis
Clinical myocardial T1 mapping protocols typically acquire three 2D slices, each obtained in a separate breath-hold; this is only feasible in patients able to do sequential breath-holds. We propose SMS-ProMyoT1 which integrates simultaneous multi-slice (SMS) with open-source ProMyoT1, previously implemented with Pulseq, to obtain all slices in a fast single shot. SMS-ProMyoT1 differs from previous implementations by combining blip-bSSFP with VERSE-multiband for RF modulation, and auto-calibrated blip patterns for a fast self-contained sequence. The method was successfully applied both in a reference phantom and in-vivo.
Introduction
Clinical myocardial T1 mapping protocols typically acquire three 2D slices1, each obtained in a separate breath-hold. Even if each slice takes only 10-15s to acquire, several minutes may be required to obtain three slices as delays are needed for the patient to recover between breath-holds. Furthermore, this is only feasible in patients able to do sequential breath-holds. We propose SMS-ProMyoT1 which integrates simultaneous multi-slice (SMS) with open-source ProMyoT12 previously implemented with Pulseq3 to obtain all slices in a fast single shot. SMS-ProMyoT1 differs from previous implementations4 by combining blip-bSSFP5 with VERSE-multiband (MB)6,7 for RF modulation, and auto-calibrated blip patterns8 for a fast self-contained sequence.Methods
SMS-ProMyoT1 was built from ProMyoT1 in Pulseq3 (Fig.1). Three methods were combined to achieve a fast bSSFP T1-mapping auto-calibrated sequence: VERSE-MB (for minimizing the achievable TR and minimizing SAR increase due to SMS), blip-bSSFP (to induce CAIPI-shifts without disturbing the bSSFP steady-state), and auto-calibration blip patterns (to ensure consistency between the data and coil sensitivity information for SMS unfolding regardless of breath-hold and cardiac phases).The MB RF pulse was modulated by applying VERSE to the single band (SB) RF pulse (t=490μs; TBP=1.5) followed by a phase optimized MB modulation to simultaneously excite 2 (SMS2) or 3 (SMS3) slices.7 Modulation was performed to obtain a slice gap (zgap) of 42 mm or 24 mm (in-vivo), with B1max=12μT, resulting in a MB RF pulse duration of 670μs and 710μs for SMS2 and SMS3, respectively (Fig.1a).
Caipi blips were added to the pre and post refocusing slice selection (SS) gradients5 for shifts of FOV/2 (-𝝅/2, 𝝅/2) and FOV/3 (-2𝝅/3, 0, 2𝝅/3) for MB factors 2 and 3, respectively (Fig1b).
Lastly, auto-calibration blip patterns were added to the centre lines of the last image, since the signal is expected to be more stable at this stage of the T1-recovery (Fig.1c). To retrieve aucocalibration information for reconstruction, 22 centre lines were acquired for each of the 2 or 3 patterns for SMS2 and SMS3. In addition to the in-plane fully sampled, an in-plane acceleration factor of 2 was also tested including 38 central lines.
SMS-ProMyoT1 (TRSMS3/TESMS3=3.14/1.57ms; TRSMS2/TESMS2=3.08/1.54ms, linear ramp-up of 11 pulses, flip angle=35º, slice thickness=6mm, FOV=200×200mm2, matrix size=128×128, zgap=42mm) was tested in a Siemens Aera 1.5 T scanner with a 20-channel head coil in the ISMRM/NIST phantom9. SB-ProMyoT1 was acquired with the same parameters except TR/TE=3.02/1.17ms, clinical MOLLI and reference tabulated phantom T1 values were used for comparison. In-vivo data of the thigh was also acquired in one healthy volunteer with an 18-channel body coil (TR/TE=3.14/1.52ms, linear ramp-up of 11 pulses, flip angle=35º, slice thickness=6mm, FOV=384×327mm2, matrix size=256×144, zgap=24mm).
Image reconstruction (inverse FFT for ProMyoT1 and split-slice GRAPPA for SMS-ProMyoT1) and T1 estimation (fitting the 3-parameter model using the function lsqcurve) were performed offline using Matlab.
The tool SAR4Seq10,11 was used prior to scanning to ensure all acquisitions complied with SAR limits (0.8W/kg for exposed body in SMS3).
Results
T1 weighted (T1w) SMS2-ProMyoT1 and SMS3-ProMyoT1 images (first inversion time (TI)) were correctly reconstructed (Fig.2). Estimated T1 maps are presented in Fig.3a. T1 estimates were similar between SMS-ProMyoT1 implementations without in-plane acceleration for high T1 values (>500ms), and underestimated for lower T1 reference values (Vial#5 T1ref=527ms; T1SMS2=462±40ms vs T1SMS3=431±66ms vs T1SB=492±34ms).T1w images and T1 maps obtained in-vivo are presented in Fig.4. Despite the smaller zgap (24mm) than phantom data, SMS reconstruction allowed to obtain T1w images without artefacts for both SMS2 and SMS3 implementations. T1 maps obtained are similar to their SB counterpart (Fig.5).
Discussion
SMS-ProMyoT1 allowed to acquire 2 and 3 slices simultaneously and correctly reconstruct them without the need for any additional calibration acquisition which could introduce errors due to motion. This was possible by including 22 extra lines in the last TI (additional 67 ms for SMS2 and 69 ms for SMS3). It was hence possible to obtain T1 maps of 3 slices in the same time as conventional mapping for one slice. This approach can be adapted to other bSSFP-based quantification methods. Adding in-plane undersampling would reduce the acquisition window and allow measurement for different heart rates, but further investigation is needed.The TR achieved for SMS-ProMyoT1 could be further reduced by increasing the B1max allowed in the VERSE optimization, reducing the minimum TI, the acquisition window, and off-resonance effects at the cost of an SAR increase.6 Although VERSE-MB allows for a more realistic SS gradient, considering the gradient impulse response could improve RF slice profile.7
Conclusion
We have implemented a fully open-source SMS bSSFP sequence including auto-calibration for fast self-contained T1-mapping. Satisfactory results were obtained with SMS2 and SMS3 both in a reference phantom and in-vivo. Further work is underway to enable joint in-plane SMS artefact-free acquisitions to allow cardiac measurements in a wider range of heart rates.Acknowledgements
Fundação para a Ciência e a Tecnologia (SFRH/BD/120006/2016, PTDC/EMD-EMD/29686/2017) e Programa Operacional Regional de Lisboa 2020 (LISBOA-01-0145-FEDER-029686).References
- Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26(4):1081-1086.
- Gaspar AS, Silva NA, Nunes RG. ProMyoT1: Open-source Inversion recovery myocardial T1 mapping sequence for fast prototyping. Proc. of Annual Meeting ISMRM 2021, Virtual Meeting, 2021.
- Layton KJ, Kroboth S, Jia F, et al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn Reson Med. 2017;77:1544-1552.
- Bentatou Z, Troalen T, Bernard M, et al. Simultaneous multi-slice T1 mapping using MOLLI with blipped CAIPIRINHA bSSFP. Magn Reson Imaging. 2020; In Press.
- Price AN, Cordero-Grande L, Malik SJ, Hajnal J V. Simultaneous multislice imaging of the heart using multiband balanced SSFP with blipped-CAIPI. Magn Reson Med. 2020;83(6):2185–96.
- Abo Seada S, Price AN, Hajnal J V, Malik SJ. Minimum TR radiofrequency-pulse design for rapid gradient echo sequences. Magn Reson Med. 2021;86(1):182–96.
- Abo Seada S, Price AN, Schneider T, Hajnal J V, Malik SJ. Multiband RF pulse design for realistic gradient performance. Magn Reson Med. 2019;81(1):362–76.
- Ferrazzi G, Bassenge JP, Mayer J, Ruh A, Roujol S, Ittermann B, et al. Autocalibrated cardiac tissue phase mapping with multiband imaging and k-t acceleration. Magn Reson Med. 2020;84(5):2429–41.
- Stupic KF, Ainslie M, Boss MA, et al. A standard system phantom for magnetic resonance imaging. Magn Reson Med. 2021; 86:1194–1211.
- Geethanath S, Kabil J, Vaughan JT. Radio frequency safety assessment for open source pulse sequence programming. eMagRes. 2020;9(1):39-48.
- imr-framework/sar4seq: To compute Specific Absorption Rate for MRI pulse sequences. Accessed July 12, 2021. https://github.com/imr-framework/sar4seq.
Figures
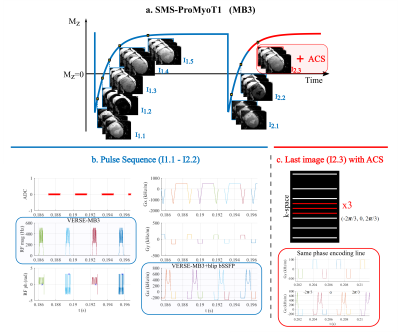
Figure 1. Scheme of SMS-ProMyoT1 sequence: a. acquisition scheme 5(3)3 where 3 slices are acquired simultaneously for each readout (MB3); b. Pulse sequence created in Pulseq where VERSE-MB3 RF pulse is applied in combination with blip bSSFP; c. in the last readout additional center lines are acquired with different blip patterns to calculate the auto-calibration signal (ACS) for SMS and in-plane undersampling reconstruction.
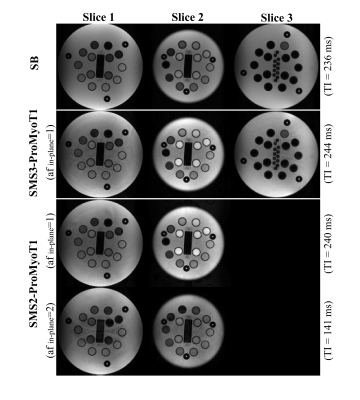
Figure 2. T1 weighted (T1w) images for the first inversion time (TI) obtained with SMS-ProMyoT1 using a slice gap of 42 mm with SMS2-ProMyoT1 and SMS2-ProMyoT1 acquisitions of the NIST/ISMRM phantom. T1w images for SMS2-ProMyoT1 are also presented with in-plane undersampling (acceleration factor (af)=2). Simulated heart rate of 50 bpm.
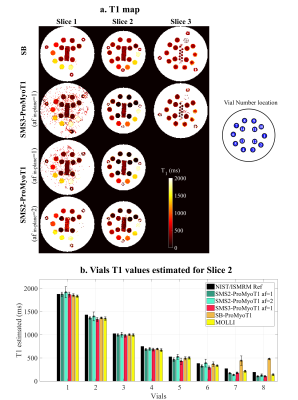
Figure 3. a. T1 maps (in ms) estimated from SMS3-ProMyoT1 and SMS2-ProMyoT1 (acceleration factor (af) in-plane 1 and 2) of NIST/ISMRM phantom. A scheme of vial number location is presented. b. T1 estimates, and respective standard deviation (SD) (in ms) obtained with 3-parameter model fitting for: SMS2-ProMyoT1 (green) and SMS3-ProMyoT1(red), with and without in-plane undersampling; and with SB-ProMyoT1 (orange) and clinical available MOLLI (yellow). Reference T1 values of NIST/ISMRM phantom are presented in black.
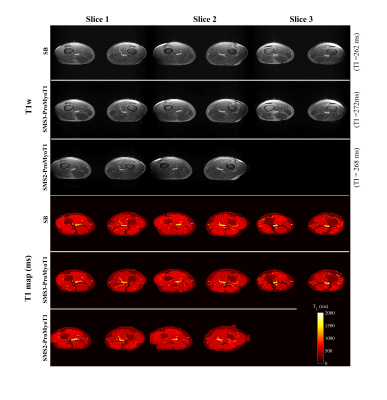
Figure 4. T1 weighted (T1w) images for first inversion time (TI) obtained with SMS-ProMyoT1 (top) in-vivo using a slice gap of 24 mm, and respectively. T1 maps (in ms) estimated from SMS2-ProMyoT1 and SMS3-ProMyoT1. SB-ProMyoT1 is also presented.
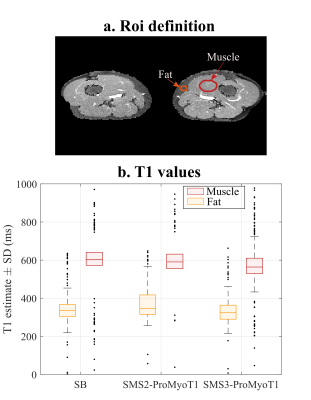
Figure 5. a. Two regions of interest (ROI) defined for in-vivo acquisition: muscle (red), and fat (orange); and respective b. T1 estimates (+/-standard deviation (SD)) (in ms) obtained with 3-parameter model fitting for SB, SMS2-ProMyoT1 and SMS3-ProMyoT1 for muscle (red) and fat (orange). SB-ProMyoT1 was shown to be comparable with clinically available MOLLI in reference 2.
DOI: https://doi.org/10.58530/2022/1340