1338
Free-Breathing R2* Mapping for Hepatic Iron Quantification Using Respiratory Motion-Resolved 3D Multi-Echo UTE Cones MRI1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of, 3GE Healthcare, Waukesha, WI, United States, 4Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Respiratory motion is one of the major factors hindering accurate R2* mapping for hepatic iron quantification. In this work, motion-resolved 3D multi-echo UTE cones MRI with pseudo-random view ordering was implemented for motion-robust and free-breathing R2* mapping of hepatic iron. Compared to conventional gridding reconstruction, motion-resolved reconstruction reduced the overestimation of R2* induced by respiratory motion artifacts, enabling an accurate measurement of R2*. 3D multi-echo UTE cones MRI with motion-resolved reconstruction demonstrated the feasibility of accurate free-breathing R2* mapping for hepatic iron quantification.
Introduction
Respiratory motion hinders accurate R2* mapping for hepatic iron quantification. Motion-robust and free-breathing R2* mapping techniques remain an unmet need. In this work, a combination of 3D multi-echo UTE cones MRI with pseudo-random view ordering1 and motion-resolved sparse reconstruction was developed for measurement of short T2* for hepatic iron overload during free-breathing. Two different motion-resolved (echo-by-echo and echo-stacked) and conventional gridding reconstructions were performed on acquired multi-echo images. Reconstructed R2* values of hepatic iron were compared with those from commercially available breath-hold 3D Cartesian multi-echo images.Methods
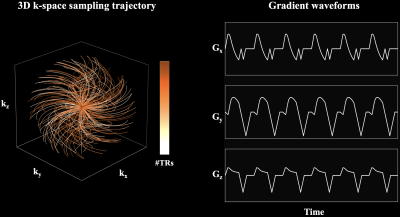
Data Acquisition and Image Reconstruction3D multi-echo UTE cones acquisition uses an RF-spoiled gradient echo sequence with the 3D cones k-space sampling trajectory2 as shown in Figure 1. A spiral-like cone interleaf traverses from the center of k-space along the conical surface and rewinds to the center of k-space. The order of cone interleaves is determined by a cyclic golden-ratio permutation scheme to improve motion robustness1.
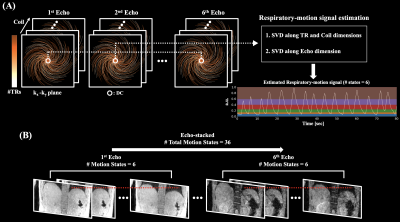
Acquired multi-echo complex source images in free-breathing condition were reconstructed using motion-averaged reconstruction (conventional gridding), and motion-resolved reconstructions. Respiratory-motion signal used in motion-resolved reconstructions was estimated as shown in Figure 2(A). Singular value decomposition (SVD) was applied to repeatedly acquired k-space direct current (DC) signal along TR and coil dimensions. Additional SVD was applied to previously estimated respiratory-motion signal along the echo dimension for the detection of improved respiratory signal. Based on the estimated respiratory-motion signal, the number of motion states was set to six such that each of the motion states has the same number of cone interleaves. Echo-by-echo motion-resolved reconstruction was performed by enforcing motion-state sparsity with total variation regularization along the motion state dimension3,4. For echo-stacked motion-resolved reconstruction, six motion states were stacked for each echo as shown in Figure 2(B). Then, motion-resolved reconstruction was performed using the multiscale low-rank matrix factorization4,5. Total number of 36 (6 motion states × 6 echoes) “motion states” were reconstructed. The regularization parameters showing the best trade-off between noise-level and image blurring were subjectively chosen for motion-resolved reconstructions. Once images were reconstructed, magnitude-based mono-exponential R2* fitting was performed.
Phantom and In-vivo Studies
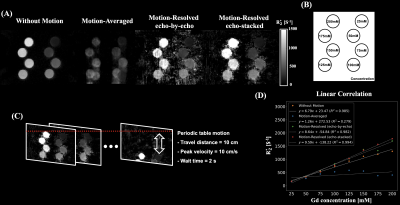
A phantom was built by diluting the gadolinium solution with saline and 1% agarose gel solution to eight concentrations ranging from 25 to 200 mM. Two 3D multi-echo UTE cones acquisitions were performed for the eight configurations. To generate motion artifacts, periodic table motion was applied to one acquisition.
With local IRB approval, two healthy subjects were scanned. All images were acquired using a 3T clinical MRI scanner (SignaPremierXT, GE Healthcare, Waukesha, WI) with a 32-ch body coil. 3D Cartesian images were acquired using a commercially available chemical-shift-encoded multi-echo spoiled gradient echo sequence (IDEAL-IQ, GE Healthcare, Waukesha, WI) with the following parameters: Matrix size = 184 (128) × 204 (162) × 36, in-plane resolution = 2.4 (2.9) × 2.2 (2.3) mm2, slice thickness = 6 mm, FA = 3°, initial TE/TE/TR = 0.728 (0.684)/0.7 (0.6)/5.7 (5.3) ms, rBW = 1275 (1562) Hz/Px, ETL/#shots = 3/2, acceleration = 4 , scan time = ~30 sec with a single breath-hold.
3D multi-echo UTE cones acquisitions were performed following parameters: Matrix size = 120 (226, 190) × 120 (226, 190) × 80 (100), in-plane resolution = 2 × 2 mm2, slice thickness = 2 mm, FA = 3°, initial TE/TE/TR = 0.032/1.3 (1.5, 1.4)/10.8 (11.5, 11.4) ms, #TEs = 6, readout duration = ~1ms, no acceleration, scan time = ~6 min with free breathing of subjects.
Results
Phantom StudyMotion-resolved reconstructions exhibited similar R2* map quality with the reference (without motion) compared to motion-averaged reconstruction as shown in Figure 3. Linear correlations of motion-resolved reconstructions were in good agreement with reference compared to motion-averaged reconstruction.
In-vivo Study
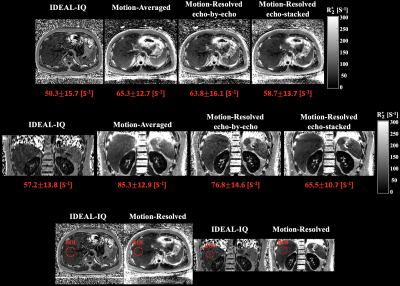
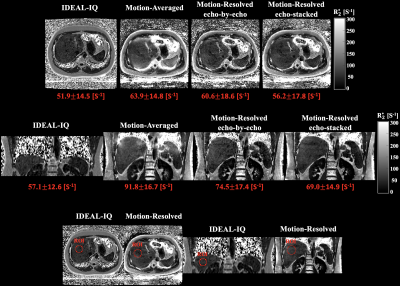
Motion-resolved reconstructions of two subjects showed comparable R2* maps with the reference (IDEAL-IQ) compared to motion-averaged reconstruction as shown in Figures 4 and 5. In ROI comparisons, echo-stacked motion-resolved reconstruction showed the smallest mean value difference compared to reference.
Discussion
This work demonstrated the feasibility of motion-robust and free-breathing R2* mapping for hepatic iron quantification using motion-resolved 3D multi-echo UTE cones MRI. R2* maps from motion-resolved reconstructions exhibited good agreement with reference R2* maps compared to motion-averaged reconstruction. More detailed comparison between two motion-resolved reconstructions will be explored to elucidate the difference between two motion-resolved reconstruction algorithms. Despite improved image quality and ROI based R2* measurement of the cones acquisition, motion-resolved reconstructions still showed overestimated R2* values compared to the reference. Further optimization of reconstruction algorithms is required as future work. The other source of overestimation may be difference in echo spacing between the reference and the cones acquisition. In order to acquire in- and out-of-phase cones data, time-interleaved acquisition will be considered. The developed method will be applied to larger data sets including patients with hepatic iron overload to determine the quantitative accuracy of motion-resolved reconstruction for a clinically relevant range of R2*.Conclusion
Motion-robust and free-breathing R2* mapping for hepatic iron quantification is feasible with motion-resolved 3D multi-echo UTE cones MRI, showing good agreement with breath-held 3D Cartesian R2* maps.Acknowledgements
No acknowledgement found.References
1. Kee Y, Sandino CM, Syed AB, Cheng JY, Shimakawa A, Colgan TJ, Hernando D, Vasanawala SS. Free‐breathing R2* mapping of hepatic iron overload in children using 3D multi‐echo UTE cones MRI. Magnetic Resonance in Medicine. 2021 May;85(5):2608-21.
2. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2006 Mar;55(3):575-82.
3. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magnetic resonance in medicine. 2016 Feb;75(2):775-88.
4. Ong F, Zhu X, Cheng JY, Johnson KM, Larson PE, Vasanawala SS, Lustig M. Extreme MRI: Large‐scale volumetric dynamic imaging from continuous non‐gated acquisitions. Magnetic resonance in medicine. 2020 Oct;84(4):1763-80.
5. Ong F, Lustig M. Beyond low rank+ sparse: Multiscale low rank matrix decomposition. IEEE journal of selected topics in signal processing. 2016 Mar 23;10(4):672-87.
Figures