1334
Developing a Delta Radiomics Framework for Prostate Cancer Progression Biomarkers in Patients under Active Surveillance: Pilot Study1Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway, 2Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 3Department of Urology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Synopsis
Identification of progressive lesions over time is important to monitor the prostate cancer progression in active surveillance. Delta Radiomics, computed as the relative changes of features among scans, provides temporal evolution of features that may indicate clinical changes along time periods. This pilot study aims to develop a framework to identify progressive lesions on two long-term scans in prostate T2WI based on Delta-radiomics. The current results show that four Delta-radiomics features from GLDM and Shape feature groups have potentials to identify progressive lesions compared to other selected reproducible features.
Introduction
T2-Weighted Imaging (T2WI) MRI is useful to visualize the anatomy of the prostate gland and lesions.1 Although tumor regions ambiguities and signal intensity are still challenges, T2WI is prospective to discriminate clinically significant prostate cancer (PCa) in Active Surveillance.2 By analyzing radiomics features over the course of observations, Delta-radiomics in T2WI provides imaging biomarkers that can be used for longitudinal monitoring of PCa progression.3 A basic requirement for Delta-Radiomics is to use reproducible and standardized features taken in short-time acquisitions to guarantee the validity for follow-up along longer time periods.4-5 The aim of this pilot study was to develop a Delta-radiomics framework to identify progressive lesions in patients under Active Surveillance.Methods
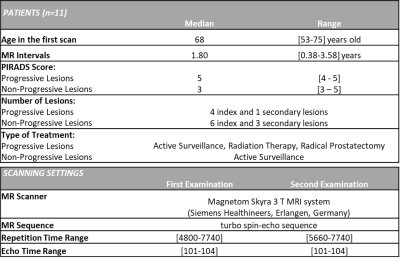
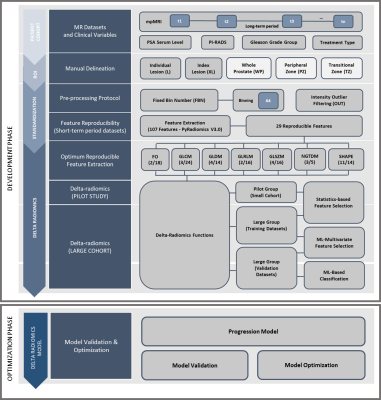
T2W images, treatment plans and clinical variables from mpMRI examinations (median interval 1.80 years, range 0.38-3.58 years) were retrospectively collected from 11 patients. The patients were examined at St. Olavs Hospital, Norway, between 2015 and 2018. The first scan was a detection or follow-up scan6, and the second was to determine treatment strategy. All patients were scored with PI-RADS≥3 and presented with at least one lesion. Patient details and scan parameters are provided in Figure 1. The proposed workflow of the Delta-radiomics framework is illustrated in Figure 2 and this pilot study was used as a testbed to see the feasibility of the implementation of the framework in small number of patient datasets from our cohort study. Individual lesions were manually segmented (n=14, 11 index and 3 secondary lesions) by a radiology resident using ITK-SNAP7 under supervision of a senior radiologist. Based on type of treatment and biopsy results information from the clinical variables, the lesion datasets were grouped into Progressive Lesions (n=5, 4 index and 1 secondary lesions) and Non-Progressive Lesions (n=9, 7 index and 2 secondary lesions). The images were pre-processed prior to feature extraction using an optimized protocol; Fixed-Bin Number (64) Discretization8, and Intensity Outlier Filtering9 to normalize the image. A prior reproducibility study10 of radiomics features was performed using two short-term T2WI scans from 49 patients with similar inclusion criteria. In this study10, 29 features selected from PyRadiomics v3.011 from six texture groups and one morphologic group were categorized as good reproducibility (ICC>0.75). Delta-radiomics features from these 29 features were computed from our current cohort study as the relative difference of feature level from the second to the first scans.12 Progressive features were determined by selecting the delta features which have significant difference between the Progressive Lesions and Non-Progressive Lesions groups that were made using two-tailed Mann-Whitney U test. Visual feature plots of the tested features in two scans from two different groups were performed to see how far the framework could identify the progression in lesions. The analysis was performed using Matlab R2020a (The MathWorks, Inc., USA).Results
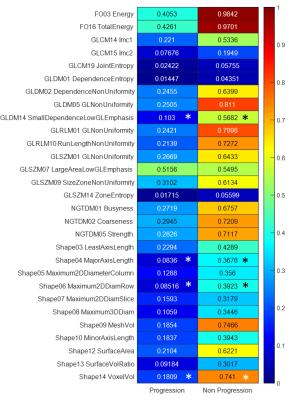
Figure 3 shows the heatmap of Delta-radiomics features in Progressive and Non-Progressive lesions groups from 29 reproducible features. Overall comparison of all features confirmed that there is significant difference between Progressive and Non-Progressive lesions (p<0.05). In feature level, comparisons between Progressive and Non-Progressive lesions groups on 29 reproducible features resulted that four Delta-radiomics features, GLDM14 Small Dependence Low Gray-Level Emphasis, Shape04 MajorAxisLength, Shape06 Maximum 2D Diameter (Row), and Shape14 Voxel Volume have significant differences (p<0.05). Visual feature plots of the significant features in Figure 4 show that many of the lesions in the same group have the same characteristics. Any outliers mostly occurred in the secondary lesions.Discussions
This study presents a framework for delta-radiomics in monitoring PCa progression for patients with PIRADS≥3 from a long-term follow-up scan. The selection of 29 good reproducible features from our previous study10 enabled trusted Delta-radiomics analysis. Based on the 29 Delta-radiomics features, indicating potential progressive feature candidates from the significant different features between Progressive and Non-Progressive lesions groups seems to be the simplest way. However, as seen from Figure 3, although four significant Delta-radiomics features, GLDM14 Small Dependence Low Gray-Level Emphasis, Shape04 MajorAxisLength, Shape06 Maximum 2D Diameter (Row), and Shape14 Voxel Volume, were obtained, the Delta-radiomics features from the Progressive group are relatively lower than those from the Non-Progressive group. In spite of that, while small datasets cannot describe the actual implementation, visual feature plots of these significant features in Figure 4 have shown the similar trends in many of the lesions. Thus, these features have the potentials to identify Progression in lesions. In this study we propose a Delta-radiomics framework to investigate prospective radiomics features that can identify progressions in longitudinal time frame. As baseline, our Delta-radiomics calculation on 29 reproducible features with optimized pre-processing protocol in short-term scans have shown to result in almost zero values. The pilot implementation of the framework on small cohort with 11 long-term datasets has provided feasibility and potential use for long-term scans. For future work, a larger cohort will be used to verify the actual performance of the framework.Conclusions
This pilot study presents a framework for detection of progressive lesions using Delta-radiomics on T2W images in long-term scans. The framework provides good indication on the utility of delta-radiomics for monitoring PCa progression in patients under active surveillance.Acknowledgements
No acknowledgement found.References
- Gupta RT, Spilseth B, Patel N, Brown AF, Yu J. Multiparametric prostate MRI: focus on T2-weighted imaging and role in staging of prostate cancer. Abdominal Radiology. 2016;41(5):831-43.
- Giganti F, Gambarota G, Moore CM, Robertson NL, McCartan N, Jameson C, Bott SR, Winkler M, Whitcher B, Castro‐Santamaria R, Emberton M. Prostate cancer detection using quantitative T2 and T2‐weighted imaging: The effects of 5‐alpha‐reductase inhibitors in men on active surveillance. Journal of Magnetic Resonance Imaging. 2018 Jun;47(6):1646-53.
- Gillies RJ, Schabath MB. Radiomics improves cancer screening and early detection. Cancer Epidemiology and Prevention Biomarkers. 2020 Dec 1;29(12):2556-67.
- Schwier M, van Griethuysen J, Vangel MG, Pieper S, Peled S, Tempany C, Aerts HJ, Kikinis R, Fennessy FM, Fedorov A. Repeatability of multiparametric prostate MRI radiomics features. Scientific reports. 2019 Jul 1;9(1):1-6.
- Zwanenburg A, Vallières M, Abdalah MA, Aerts HJ, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020 May;295(2):328-38.
- Lo GC, Margolis DJ. Prostate MRI with PI-RADS v2. 1: initial detection and active surveillance. Abdominal Radiology. 2020 Jul;45(7):2133-42.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006 Jul 1;31(3):1116-28.
- Duron L, Balvay D, Vande Perre S, Bouchouicha A, Savatovsky J, Sadik JC, Thomassin-Naggara I, Fournier L, Lecler A. Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS One. 2019 Mar 7;14(3):e0213459.
- Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magnetic resonance imaging. 2004 Jan 1;22(1):81-91.
- Dewi DEO, Sunoqrot MRS, Nketiah GA, Sandsmark E, Langørgen S, Bertilsson H, Elschot M, and BathenTF. Repeatability of Radiomic Features in T2-Weighted Prostate MRI: Impact of Pre-processing Configurations. 2021 ISMRM & SMRT Annual Meeting. 2021 May.
- Van Griethuysen JJ, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RG, Fillion-Robin JC, Pieper S, Aerts HJ. Computational radiomics system to decode the radiographic phenotype. Cancer research. 2017 Nov 1;77(21):e104-7.
- Chang Y, Lafata K, Sun W, Wang C, Chang Z, Kirkpatrick JP, Yin FF. An investigation of machine learning methods in delta-radiomics feature analysis. PloS one. 2019 Dec 13;14(12):e0226348.
Figures
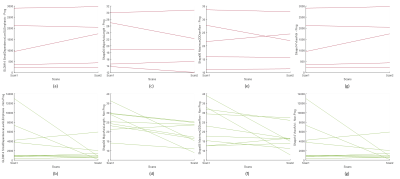
Visual feature plots of the four selected significant features:
(a) GLDM14 progressive:3 increasing, 1 decreasing, 1 stable
(b) SHAPE04 progressive: 2 increasing, 2 decreasing, and 1 stable
c) SHAPE06 progressive: 2 increasing and 3 decreasing
(d) SHAPE14 progressive: 3 increasing, 2 decreasing, and 1 stable
(e) GLDM14 non-progressive: 2 increasing, 5 decreasing, and 2 stable
(f) SHAPE04 non-progressive: 1 increasing and 8 decreasing
(g) SHAPE06 non-progressive: 2 increasing and 7 decreasing
(h) SHAPE14 non-progressive: 2 increasing, 5 decreasing, and 1 stable