1327
Structural and functional brain imaging in a rat model of fetal growth restriction1Division of Development and Growth, Department of Paediatrics and Gynaecology-Obstetrics, School of Medicine, University of Geneva, Geneva, Switzerland, 2Center for Biomedical Imaging, Animal Imaging Technology section, Federal Institute of Technology of Lausanne, Lausanne, Switzerland, 3Division of Neonatology and Pediatric Intensive Care, Children's University Hospital of Geneva, Geneva, Switzerland, 4INSERM UMR1141 NeuroDiderot, University Paris Diderot, Sorbonne Paris Cité, Paris, France
Synopsis
Brain injuries and subsequent neurodevelopmental impairments observed following Fetal Growth Restriction (FGR) were shown to be associated with an exacerbated neonatal neuro-inflammation. The aim of this work was to characterize a rat model of FGR by advanced diffusion MRI (DTI and NODDI) and functional ultrasounds (fUS). We show in this study that neonatal inflammation lead to transient microstructural damages and subsequent behavioral and functional disabilities at long term. This model mimics very closely clinical reality in babies born following FGR. Innovative neuroimaging tools associated with this neuro-inflammatory model pave the way for their translational use in human neonates.
Introduction
Fetal Growth Restriction (FGR) is one of the most common adverse antenatal conditions in newborns, leading to a significant increase in perinatal mortality, neurological handicaps and chronic diseases in adulthood1. The brain injuries and the subsequent neurodevelopmental impairments observed in children born after FGR seem to be closely associated with an exacerbated neonatal neuro-inflammation2. In this context, advance in early life neuroimaging in animal model of FGR associated with neuro-inflammation represents major opportunity for diagnosis, prognosis and targeted therapeutic strategies. The aim of this work was to characterize a rat model of FGR by advanced diffusion MR imaging (Diffusion Tensor Imaging and Neurite Orientation Dispersion and Density Imaging) at 9.4T and functional ultrasounds (fUS).Materials and methods
Pregnant Sprague Dawley dams were exposed to a normal 23% protein diet (control diet) or an isocaloric 9% protein diet (LPD diet) during pregnancy. At birth, pups from LPD diet were daily i.p. injected with IL-1β (20 µg/kg/injection) and pups from control diet injected with Saline at post-natal day 1 and 2 (P1 and P2), resulting on two groups: Sham (control diet + saline injection) and LPD+IL-1β.MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Agilent) equipped with 12-cm gradient coils (400mT/m, 120µs) with a 2.5 mm diameter birdcage coil. At P10, P20 and P55 (9/group/age) brains were formalin fixed for subsequent MRI. A multi-b-value shell protocol was acquired using a spin-echo sequence with 3 averages and TE/TR = 45/2000 ms resulting in an image resolution of 160×160×600 µm2. A total of 96 DWI were acquired, 15 of them as b0 reference images. The remaining 81 were separated in 3 shells with the following distribution (# of directions/b-value in s/mm2): 21/1750, 30/3400 and 30/5100. All 81 directions were non-collinear and uniformly distributed in each shell.
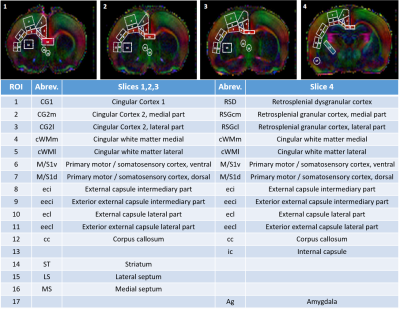
Diffusivities (Mean, MD; Axial, AD and Radial, RD) and fractional anisotropy (FA) were derived from the tensor by using DTI-TK. Acquired data were fitted using the NODDI toolbox3 leading to intra-neurite volume fraction (fin), cerebrospinal volume fraction (fiso) and orientation dispersion index (ODI). 17 different brain regions were analyzed in white matter and grey matter (Figure 1). For statistics, a Mann Whitney test was used (significance: *P<0.05).
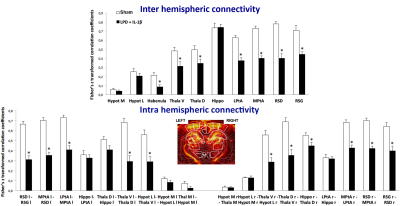
At P30 on another bench of animals (9/group), we used in-vivo high-resolution functional UltraSound (fUS) as previously described4 to generate highly sensitive cerebral blood volume maps underlying neuronal activity in order to assess inter- and intra- hemispheric cortical functional connectivity.
Results
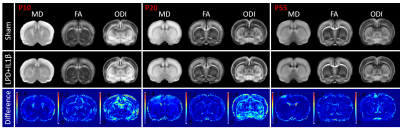
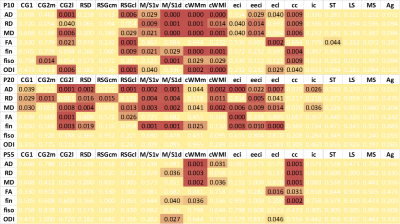
Mean maps over the groups as well as maps of differences for diffusion indices are presented in figure 2. Axial and radial diffusivities (AD & RD) and fractional anisotropy (FA), were found significantly reduced in LPD+IL-1β animals in most of the observed structures (cingular cortex and white matter, somatosensory cortex dorsal, corpus callosum and external capsule). Conversely, intra-neurite volume fraction (fin) and orientation dispersion index (ODI) were found significantly increased (Figure 3). Notice that in this case, increase of fin (commonly referred as neurite density) may represent thickening of the myelin sheath leading to increased intra-axonal diameter. Main differences in DTI and NODDI derived indices were observed at P10 and P20 whereas at P55 most of the regions recovered normal values without difference with the control group. fUS at P30 imaging revealed that FGR was associated with a significant reduction in intra- and inter-hemispheric connectivity in most of the explored cortical area (Figure 4).Discussion
This rat model of FGR associated to early postnatal neuro-inflammation recapitulated microstructural and functional cortical alteration readily observable by advanced neuroimaging tools. Increased pro-inflammatory microglia reactivity at P2 & P4 was found leading to a reduced myelination at P10 and cognitive and behavioral disorders in young adults’ animals (P21 to P55). Microstructural damages observed early (P10 and P20) are mostly recovered at P55 probably due to intrinsic defense mechanisms during brain development and referred as cerebral plasticity. Nevertheless, at longer term some behavioral and functional disabilities persisted as already shown in this model4,5.Conclusion
We show in this study that neonatal inflammation induced by antenatal FGR leads to transient microstructural damages and long-term behavioral and functional disabilities. Innovative neuroimaging tools associated with this neuro-inflammatory model pave the way for their translational use in human neonates. Finally, they provide novel insights to better monitor new neuroprotective candidates and assess the global impact of neuro-inflammation in the immature brain.Acknowledgements
Supported by the CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, Leenards and Jeantet foundation.References
(1) Guellec, I., Marret, S., Baud, O., et al. (2015). Intrauterine growth restriction, head size at birth, and outcome in very preterm infants. The Journal of Pediatrics, 167(5), 975–981.e972. https://doi.org/10.1016/j.jpeds.2015.08.025.
(2) Hagberg, H., Gressens, P., & Mallard, C. (2012). Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Annals of Neurology, 71(4), 444–457. https://doi.org/10.1002/ana.22620.
(3) Zhang, H., Schneider, T., Wheeler-Kingshott. C.A., et al. (2012). NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016.
(4) Rideau Batista Novais, A., Pham, H., van de Looij, Y., et al. (2016). Transcriptomic regulations in oligodendroglial and microglial cells related to brain damage following fetal growth restriction. Glia 64(12):2306–20.
(5) Mairesse, J., Zinni, M., Pansiot, J., et
al. (2019) Oxytocin receptor agonist reduces perinatal brain damage by
targeting microglia. Glia 67(2):345–59.
Figures