1326
Region-specific effects of neonatal hyperbilirubinemia on the developing brain in a preterm Gunn rat model1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States
Synopsis
Preterm Gunn rat model of neonatal hyperbilirubinemia (NHB) was used to investigate effects of unconjugated bilirubin (UCB) on the developing hippocampus and cerebellum. In this model, sulfadimethoxine was injected (i.p.) to Gunn rat pups on postnatal day 5 to exaggerate NHB effects at a preterm equivalent age. 1H MRS and MRI results clearly demonstrate that high levels of UCB in early postnatal age critically affects the brain development in a region-specific manner. In this model, cerebellum appears to be much more vulnerable to high levels of UCB than hippocampus resulting in abnormal postnatal development.
INTRODUCTION
Neonatal hyperbilirubinemia (NHB) can lead to devastating brain injury in newborn infants.1 High levels of unconjugated bilirubin (UCB) are toxic to the cerebellum, hippocampus, basal ganglia and auditory pathways; leading to cerebral palsy, hearing loss, and a range of cognitive and behavioral deficits.2 Preterm infants are more vulnerable to bilirubin toxicity, but the exact mechanism and extent of injury in this population is not well understood.3,4 The Gunn rat model of NHB is a well-established model of bilirubin encephalopathy,5 but is more commonly used at a full-term human brain equivalent age. Gunn rats have a spontaneous deficiency in the UDP-glucuronyl transferase enzyme, which is responsible for conjugating bilirubin in the liver. Homozygous Gunn rat pups (jj) develop an unconjugated hyperbilirubinemia due to minimal activity of this enzyme. Sulfadimethoxine can be used in preterm Gunn rat model to displace UCB from albumin, thus increasing UCB transfer across the blood brain barrier, leading to brain injury. However, the effects of exaggerated NHB on the preterm brain regions are poorly understood. We sought to study Gunn rat pups during a developmental time window that more closely corresponds to the preterm brain, and to compare bilirubin-induced injury between the hippocampus and cerebellum, two regions known to be affected by both bilirubin and prematurity. The purpose of this study was to investigate neurochemical changes in the hippocampus and cerebellum induced by NHB using 1H MRS and the Gunn rat pup model.METHODS
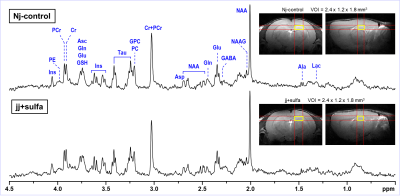
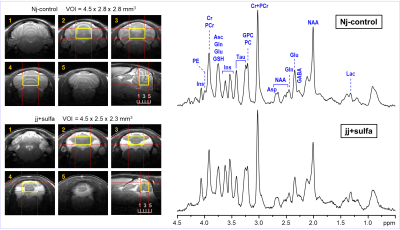
Jaundiced pups (jj+sulfa, N = 8) were injected with sulfadimethoxine (200 mg/kg, i.p.) on postnatal day 5, a timepoint that approximates the brain development of an extremely preterm human infant (28 weeks gestational age). Heterozygous littermates were jaundice-free and served as controls (Nj-control, N = 8). These two groups of rats were scanned by MRI/MRS on postnatal day 30 (equivalent to a toddler age of human brain). In vivo 1H MR spectra were acquired at 9.4 T from the hippocampus and cerebellum on postnatal day 30. Spontaneously breathing animals were anesthetized with 1.0 – 1.5% isoflurane and the body temperature was maintained at 370C. 1H MRS data were collected at 9.4T using FASTMAP B0 shimming 6 and LASER localization sequence 7 (TE = 15 ms, TR = 5 s) combined with VAPOR water suppression 8. Multislice FSE technique was used for imaging (slice thickness = 0.8 mm, ETL = 8, ESP = 12 ms). Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules included in the basis set. Unsuppressed water signal was used as an internal reference for MRS data acquired from the hippocampus (jj+sulfa, Nj-control) and cerebellum (Nj-control) assuming 80% brain water content. MRS data acquired from the cerebellum of jj+sulfa group were scaled to the macromolecule (MM) content using the average MM level of the Nj-control group.RESULTS
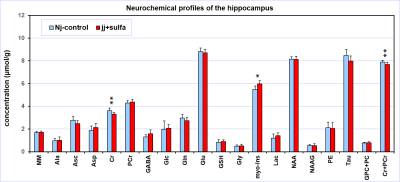
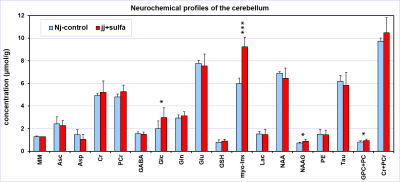
The spectral quality consistently achieved in this study (Figs. 1, 2) allowed high precision of neurochemical profiling (Figs. 3, 4). Due to increased microscopic B0 heterogeneity, the spectral linewidth in the cerebellum was about 7 Hz larger than in the hippocampus despite the same quality of FASTMAP B0 shimming. 1H MRS data acquired from the hippocampus of jj+sulfa group showed significant but relatively small changes in creatine (-8%, p = 0.010), total creatine (-3%. p = 0.029) and myo-inositol (+9%, p = 0.004) relative to Nj-control group (Fig. 3). In addition, a trend for increased PCr/Cr ratio (+10%, p = 0.066) was observed. There were no morphological changes in the hippocampus. In contrast, MRI revealed major developmental deficit in the cerebellum of jj+sulfa rats (Fig. 2). While, the size of VOI was reduced for jj+sulfa group, acquired spectra were affected by the partial volume effect caused by non-cerebellar volume (CSF) included in the VOI. Therefore, cerebellar metabolite concentrations were normalized to MM. The cerebellar neurochemical profile of the jj+sulfa group was considerably different from the Nj-control (Fig. 4). Myo-inositol was increased by 54% (p < 0.0001) and glucose by 51% (p = 0.036) in jj+sulfa rats relative to Nj-controls. In addition, increased levels of N-acetylaspartylgbutamate (NAAG, +21%, p = 0.032) and the sum of glycerophosphocholine and phosphocholine (GPC+PC, +17%, p = 0.049) were observed in jj+sulfa group.DISCUSSION
1H MRS and MRI findings in this preterm Gunn rat model (Figs. 3, 4) clearly demonstrate that high levels of UCB critically affects brain development in a region-specific manner. Signs of abnormal brain developmental resulting from NHB were predominantly observed in the cerebellum of jj+sulfa rats. Large changes in myo-Ins indicate osmotic imbalance. Higher glucose and PCr/Cr ratio and lower Cr suggest decreased demands for energy due to suppressed neuronal activity. Changes in NAAG and GPC+PC may indicate altered myelination. The results of this study demonstrate that this animal model of NHB is promising in providing new information about the mechanism and impact of UCB on the developing brain, which may potentially lead to the development of new treatment strategies.CONCLUSION
The preterm Gunn rat model revealed that the cerebellum is the brain region most vulnerable to high levels of unconjugated bilirubin resulting in abnormal postnatal development.Acknowledgements
Supported by: NIH grants P41 EB027061 and P30 NS076408, Progressive Grant from the Department of PediatricsReferences
1. Stevenson DK, Maisels MJ, Watchko J. Care of the Jaundiced Neonate. New York: McGraw-Hill Medical; 2012.
2. Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. N Engl J Med. 2013;369(21):2021-2030.
3. Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012;32(9):660-664.
4. Watchko JF, Maisels MJ. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin Perinatol. 2014;38(7):397-406.
5. Chowdhury JR, Kondapalli R, Chowdhury NR. Gunn rat: a model for inherited deficiency of bilirubin glucuronidation. Adv Vet Sci Comp Med. 1993;37:149-173.
6. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319-323.
7. Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155-177.
8. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649-656.
Figures