1324
Dose-dependent neuroprotective effects of Bovine Lactoferrin following neonatal hypoxia-ischemia in the immature rat brain1Division of Development and Growth, Department of Paediatrics and Gynaecology-Obstetrics, School of Medicine, University of Geneva, Geneva, Switzerland, 2Center for Biomedical Imaging, Animal Imaging Technology section, Federal Institute of Technology of Lausanne, Lausanne, Switzerland
Synopsis
Injuries to the developing brain due to hypoxia–ischemia (HI) are common causes of neurological disabilities in preterm babies Lactoferrin (Lf) is an iron-binding protein shown to have antioxidant, anti-inflammatory and anti-apoptotic properties when administered to mothers as a dietary supplement during pregnancy and/or lactation in preclinical studies of developmental brain injuries. Here, we tested three different doses of dietary maternal Lf supplementation using the postnatal day 3 HI model and evaluated the acute neurochemical damage profile using 1H MRS and DTI/NODDI. In conclusion, Lf supplementation attenuates, in a dose-dependent manner, the acute and long-term cerebral injury caused by HI.
Introduction
Injuries to the developing brain due to hypoxia–ischemia (HI) are common causes of neurological disabilities in preterm babies. HI, with oxygen deprivation to the brain or reduced cerebral blood perfusion due to birth asphyxia, often leads to severe brain damage and sequelae. Injury mechanisms include glutamate excitotoxicity, oxidative stress, blood brain barrier dysfunction and exacerbated inflammation. Nutritional intervention is emerging as a therapeutic alternative to prevent and rescue brain from HI injury. Lactoferrin (Lf) is an iron-binding protein present in saliva, tears and breast milk which has been shown to have antioxidant, anti-inflammatory and anti-apoptotic properties when administered to mothers as a dietary supplement during pregnancy and/or lactation in preclinical studies of developmental brain injuries. However, despite Lf’s promising neuroprotective effects, there is no established effective dose. Here, we tested three different doses of dietary maternal Lf supplementation using the postnatal day 3 HI model and evaluated the acute neurochemical damage profile using 1H Magnetic Resonance Spectroscopy (MRS) and long-term microstructure alterations using advanced diffusion imaging (DTI/NODDI) allied to protein expression and histological analysis.Materials and methods
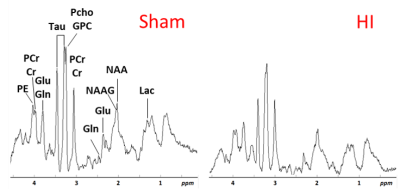
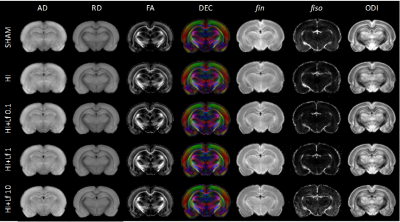
Pregnant Wistar rats were fed either control diet or bovine Lf supplemented chow at 0.1, 1 or 10 g/kg/body weight concentration from the last day of pregnancy (embryonic day 21 - E21) to weaning. rat pups were exposed to Lf through lactation. At postnatal day 3 (P3), pups had their right common carotid artery permanently occluded and were exposed to 6% oxygen for 30min. Sham rats had the incision but neither surgery nor hypoxia episode. All MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Varian/Magnex) equipped with 12-cm gradient coils (400mT/m, 120µs). A quadrature transceive 20-mm surface RF coil was used for 1H-MRS. 24h following HI, after automatic FASTMAP shimming, spectra acquisition on a VOI of 1.5×1.5×2.5mm3 within the cortical lesion was performed using an ultra-short echo time (TE/TR = 2.7/4000ms) SPECIAL spectroscopy method1. At P4, 16 series of FIDs (16 averages each) were acquired, individually corrected for frequency drift, summed together and corrected for residual eddy current effects using the reference water signal. Proton spectra were analyzed with LCModel2. At P25, rats were sacrificed and brains were paraformaldehyde-fixed for subsequent ex-vivo MRI with a 2.5 mm diameter birdcage coil. A multi-b-value shell protocol was acquired using a spin-echo sequence (FOV = 21×16mm2, matrix size = 128×92, 12 slices of 0.6mm, 3 averages with TE/TR = 45/2000ms). 96 DWI were acquired, 15 b0 images and 81 separated in 3 shells (non-collinear and uniformly distributed in each shell) with (# of directions/b-value in s/mm2): 21/1750, 30/3400 and 30/5100. Acquired data were reconstructed with DTI-TK and fitted using the NODDI toolbox3. Brain regions identified were cortex (Cx - Motor ans Somato), corpus callosum (CC) and external capsule (EC). DTI derived parameters (Axial diffusivity (AD), Radial diffusivity (RD), Mean diffusivity (AD) and Fractional anisotropy (AD)) as well as NODDI derived parameters (intra-neurite volume faction (fin), isotropic volume fraction (fiso) and orientation dispersion index (ODI)) were averaged in the different regions assessed. For statistics, one-way ANOVA followed by Duncan’s post hoc test was used (significance: P<0.05). At P4 and P25, histological analysis and protein expression were assessed in the cortex and hippocampus.Results
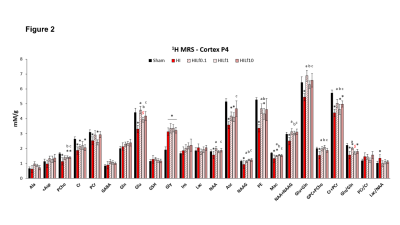
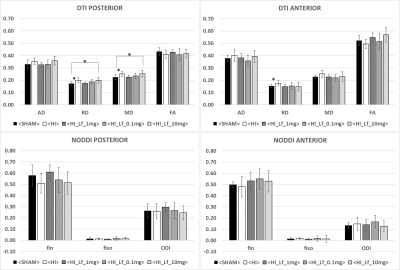
The average signal-to-noise ratio calculated on all acquired spectra (figure 1) was 12.3±2.2. HI induced a significant decrease in the concentrations of PCho, Cr, PCr, Glu, NAA, Asc, NAAG, PE, Mac, Tau, NAA+NAAG, Glu+Gln, GPC+PCho, Cr+PCr and the Glu/Gln ratio (figure 2), similar to previously reported data4. Lf supplementation through lactation was able to partially reverse most of the alterations induced by HI insult with some differences as a function of the dose. Diffusion indices showed alteration (Figure 3) in the external capsule of HI rats compared to Sham (Increased AD and RD), this difference was also observed for the Sham compared to HILf10 groups but not for the others. Similar changes were observed in the CC. No difference was observed in the cortex (Data not shown). Hitological analysis and protein expression (data not shown) also showed a neuroprotective effect dependent of the Lf dose with the dose of 1 g/kg more effective than the others.Discussion
Acute metabolic disturbance induced in cortical tissue by HIP3 was reversed with all 3 doses of Lf. However, data obtained from MRS shows that Lf neuroprotective effects were modulated by the dose. Indeed, the white matter induced lesions observed in the HI group were reversed with doses of 0.1 g/kg and 1 g/kg whereas protein expression and histological analysis illustrate the optimal effect with a dose of 1 g/kg.Conclusion
In conclusion, Lf supplementation attenuates, in a dose-dependent manner, the acute and long-term cerebral injury caused by HI, restoring brain metabolism and microstructure. From our current data, Lf reached its optimal effects with dose of 1g/kg whereas 10 g seems deleterious for some aspects. Further investigations are in progress to better understand effects of Lf.Acknowledgements
The study was funded by the Bill and Melinda Gates Foundation Grand Challenges and supported by the CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, Leenards and Jeantet foundation.References
[1] Mlynarìk V et al. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn. Reson. Med. 2006 Nov;56(5):965-70.
[2] Provencher SW et al. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993 Dec;30(6):672-9.
[3] Zhang H et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012 Jul 16;61(4):1000-16.
[4] van de Looij Y et al. Longitudinal MR assessment of hypoxic ischemic injury in the immature rat brain. Magn Reson Med. 2011 Feb;65(2):305-12.
Figures