1315
A simplified method to estimate blood flow velocity and inversion efficiency in the major cerebral arteries using multi-PLD pCASL
Freya Allery1,2, Daniel P. Bulte1, Embla Hocking1, Nicholas P. Blockley3,4, James W. Harkin5, and Joana Pinto1
1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Institute of Health Informatics, University College London, London, United Kingdom, 3Sir Peter Mansfield Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 4School of Life Sciences, University of Nottingham, Nottingham, United Kingdom, 5Poole Hospital NHS Foundation Trust, Poole, United Kingdom
1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Institute of Health Informatics, University College London, London, United Kingdom, 3Sir Peter Mansfield Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 4School of Life Sciences, University of Nottingham, Nottingham, United Kingdom, 5Poole Hospital NHS Foundation Trust, Poole, United Kingdom
Synopsis
ASL inversion efficiency is often assumed as a pre-defined value or estimated by acquiring an additional phase-contrast MR image. While the former approach might be inaccurate in certain conditions, the latter can be time-consuming. Here, we propose a simplified method that estimates blood flow velocity and inversion efficiency solely using the ASL data. This novel strategy was tested using multi-PLD pCASL datasets with two different blood flow dynamics (normo- and hypercapnia). Our results show the feasibility of this approach and highlight the need for subject/condition-specific inversion efficiency estimation for a more accurate quantification of CBF.
Introduction
When quantifying CBF using ASL imaging techniques, inversion efficiency is often assumed as a pre-defined value (0.9 for PASL and 0.85 pCASL1). Nevertheless, if blood flow velocity is altered due to a disease-related impairment or external manipulation (e.g., hypercapnia2) using this assumed value might prove to be inaccurate, compromising CBF quantification. This issue is usually circumvented by acquiring additional phase-contrast data to estimate blood velocity in major arteries, but this approach can be time-consuming and not feasible in certain circumstances. In this work we propose a novel method to estimate blood flow velocity and inversion efficiency simply based on the spatiotemporal dynamics of multi-PLD ASL, without the need to collect additional MR data.Methods
10 healthy subjects (5M, 20.4 ± 0.8 years old) were studied on a 3T Siemens Prisma Scanner. Data acquired included multi-PLD pCASL datasets (2D multi-slice GE-EPI, background suppressed, 3.5 × 3.5 × 4.5 mm3, TR/TE = 4100/14 ms, 24 slices, bolus duration 1400 ms, PLDs: 250, 500, 750, 1000, 1250,1500 ms) acquired during two conditions (6 min 40 s, each): air (normocapnia) and hypercapnia (RespirAct Gen 3, Thornhill Medical, target PETCO2 = 10 mmHg above subject’s baseline). A high-resolution structural image was also collected (MPRAGE, 1mm isotropic, TR/TE = 1900/3.74 ms).All data were analyzed using FSL and MATLAB. A standard kinetic model with dispersion3 and macrovascular4 components was fitted to the multi-PLD pCASL data using BASIL, estimating CBF and arterial arrival time in tissue (ATT) as well as in macrovascular regions (ATTa). Major arteries were identified in the derived ATTa maps for both conditions and an average ATTa value for the right-hand side middle cerebral artery (MCA) was computed for each subject/condition (ATTMCA). This value was then used to extrapolate the corresponding blood flow velocity in the internal carotid artery (ICA) using an exponential model of distance travelled as a function of ATT (d = a (e^(bt) - 1)). The gradient represents the decreasing velocity across the vascular tree, and where s is the distance from a specific point along the blood flow trajectory, t is the AAT of that same point, and a and b are the fitting parameters. At the initial point, s = 0, t = 0 and the gradient is not fixed as this gradient represents the instantaneous velocity at this point according to the model.
At a more distant point, aROI, s = saROI and t = AATaROI and the gradient is fixed at ds/dt (AATaROI) = Vc, as the velocity at this point will be equal to the velocity in the capillaries (optimised for oxygen extraction, Vc = 0.79 ± 0.03 mm/s 5). This aROI was previously obtained after segmenting the GM according to the corresponding major cerebral arteries that feed those regions (using a registered MNI152 standard atlas), yielding a specific region-of-interest fed by the MCA. The estimated ICA velocities were then used in a pre-existing pCASL inversion efficiency model that yields the inversion efficiency as a function of velocity in the ICA6.
Results and Discussion
Figure 1 shows an illustrative representation of the manual identification of the MCA region in the subject and condition-specific (b-normocapnia, c-hypercapnia) ATTa maps. These voxels were more easily identified in the normocapnia (b) rest condition. Figure 2 provides the estimated velocities in the internal carotid artery for the two conditions, with hypercapnia increasing blood flow velocities across all subjects. Figure 3 shows the pre-existing pCASL inversion efficiency model as a function of velocity in the ICA6. Figure 4 shows the corresponding inversion efficiencies, and highlights the different efficiencies between conditions, with hypercapnia having significantly lower values. These velocity and inversion efficiency values account for both subject and condition variability, possibly leading to a more accurate CBF quantification.Conclusion
Our results introduce a novel method that estimates blood flow velocities and inversion efficiency using multi-PLD pCASL data. This can be achieved for each vascular territory, possibly providing useful information in pathology or vascular irregularities. This strategy was tested in two datasets with different blood flow dynamics (normo- and hypercapnia). In the future, we plan to validate these results against blood flow velocities and inversion efficiencies derived from phase-contrast MR imaging.Acknowledgements
Acknowledgements: This work was funded by EPSRC grants EP/G004277/1, EP/K025716/1 and EP/S021507/1References
- Alsop DC, et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102-16.
- Aslan, S., et al. (2010). Estmation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn. Reson. Med 63, 765–771.
- Chappell, M. A., et al. (2013). Modeling dispersion in arterial spin labeling: validation using dynamic angiographic measurements. Magn. Reson. Med. 69, 563–70.
- Chappell, M. A., et al. (2010). Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. Magn. Reson. Med. 63, 1357–65.
- Ivanov, K. P., et al. (1981). Blood flow velocity in capillaries of brain and muscles and its physiological significance”. Microvascular Research 22.2, 143–155.
- Thomas W. Okell. “Assessment of collateral blood flow in the brain using magnetic resonance imaging”. PhD thesis. 2011.
Figures
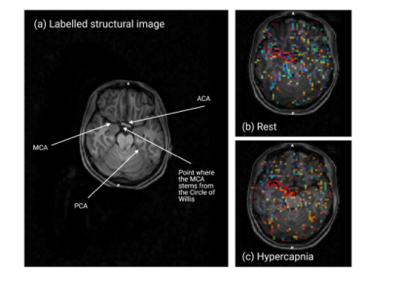
Figure 1. Structural image and macro-vascular AAT (AATa) maps for the normo- and hypercapnic conditions for an illustrative subject. Voxels corresponding to the right-hand side MCA are highlighted.
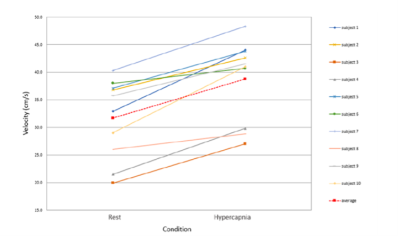
Figure 2. Average velocity in the internal carotid artery in both conditions (normocapnia and hypercapnia), estimated from the vascular region fed by the right-hand side MCA. All subjects (different colours) show the expected velocity increase from normocapnia to hypercapnia. The red dashed line shows the average velocity values and trend.
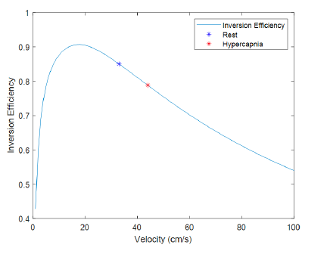
Figure 3 pCASL inversion efficiency as a function of average blood velocity through the labelling plane6. Blue and red dots represent results for an illustrative subject during the two conditions.

Figure 4. The derived inversion efficiencies plotted for each subject for the normocapnia (blue) and hypercapnic (red) conditions. The inversion efficiencies were lower in hypercapnia for all subjects. The default efficiency is plotted as the vertical green dashed line.
DOI: https://doi.org/10.58530/2022/1315