1312
Towards non-invasive assessment of cardiovascular physiology by combining cardiac MRI with predictive biomechanical modeling1UT Dallas, Dallas, TX, United States, 2Pediatrics, UT Southwestern Medical Center, Dallas, TX, United States, 3Inria, Palaiseau, France, 4LMS, Ecole Polytechnique, Palaiseau, France
Synopsis
Biomechanical models coupled with cardiovascular MRI (CMR) have the potential to provide non-invasively physiological parameters of clinical interest. The approach is presented and validated on a group of 10 patients who underwent CMR and invasive catheterization. Patient-specific models are built based on CMR data and maximum/minimum arterial pressure. The stroke work, derived from ventricular pressure-volume (P-V) loops, was comparable between the measured and simulated P-V loops, suggesting the high quality of the in silico loops. An excellent correlation of the model-derived myocardial contractility with maximum time-derivative of ventricular pressure gives an opportunity for the non-invasive characterization of patients’ inotropic state.
Introduction
Clinical management benefits from assessing detailed cardiovascular physiology and the predictions of therapeutic actions.Myocardial energetics can be obtained from the analysis of ventricular pressure-volume (P-V) loops. While cardiovascular MRI (CMR) provides accurate volumes, the invasive acquisition of ventricular pressure represents a limitation. Phenomenological models based on the so-called time-varying elastance TVE1 can be used to reconstruct P-V loops from CMR2,3 and subsequently estimate e.g. stroke work (SW, area encompassed by the loop). However, their predictive capability is limited.
Truly biophysical models have, in addition, the potential to predict the action of certain drugs; quantify tissue physiological state (e.g. myocardial contractility); and can be applicable for more complex cases (e.g. valvular defects, for which TVE models are not designed). Moreover, while every type of data is encumbered by noise, the physical character of such models gives the potential to augment the data quality by physics-based filtering.
In this work, we demonstrate using a biophysical modeling framework of complexity compatible with clinical environment4,5 to create patients’ “digital twins” from non-invasive data, in order to obtain high-quality P-V loops and to quantify the myocardial contractility. We validate the framework using the invasive catheter data.
Methods
2.1 Data acquisition:Datasets of 10 patients with tetralogy of Fallot after surgical repair (rTOF) were included in this study. The patients underwent CMR and invasive LV catheterization. Maximum and minimum aortic pressures were extracted from the waveform, and together with end-diastolic and end-systolic volumes (EDV, ESV) used to set up the patient-specific models.
2.2 Biophysical model of heart:
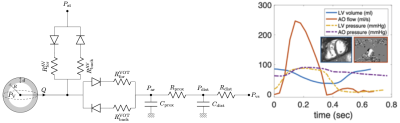
The biomechanical model of a single heart cavity was employed6,4,5. The geometry and kinematics are reduced to a sphere, while the constitutive laws are preserved as in the full 3D model7. The circulation is represented by Windkessel model, which is coupled to the heart via system of diodes representing the valves (Figure 1). The passive myocardial stiffness was adjusted in patient-specific sense according to the measured EDV and with the assumed end-diastolic pressure of 10mmHg. The circulation model was calibrated according to the aortic flow and pressure (maximum and minimum). The myocardial contractility (the active stress developed by the model during the cardiac cycle) was adjusted according to ESV.
2.3 Comparison with measurements:
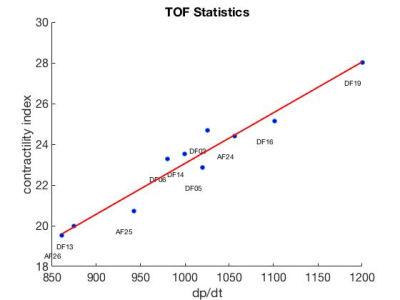
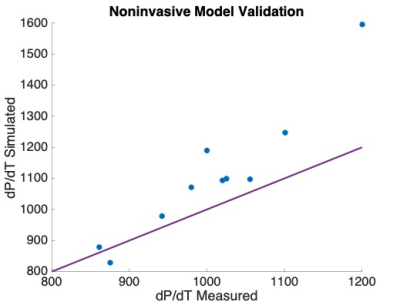
Time-vs-volume (obtained from CMR analysis8) and invasively measured LV pressure waveforms were time-synchronized9. SW from the measured data was compared with SW obtained from the simulated P-V loop using two-tailed sample t-test at p<0.05. A correlation between the model-derived contractility (indexed with respect to the size of ventricle10) and maximum time-derivative of measured LV pressure max(dp/dt) was created. Finally, the measured vs. simulated max(dp/dt) were compared using two-tailed sample t-test at p<0.05.
Results
Figure 2 demonstrates an example of simulated vs. measured cardiac cycle of a selected patient. In all 10 patients, there was no significant difference between SW of the simulation and validation data (p>0.6). The contractility and measured max(dp/dt) have a strong correlation with an R2 = 0.98 (Figure 3). There was no significant difference between the simulated and measured max(dp/dt) (p>0.3), Figure 4.Discussion
The comparison of simulated vs. measured SW suggests that the in silico derived P-V loops are an adequate alternative to those created using invasive measurements. As max(dp/dt) is a usual surrogate of contractility, the strong correlation with the model-derived contractility justifies its utility as a measure of heart inotropic state derived from non-invasive data. Finally, replacing the max(dp/dt) derived from the measured data by the value of max(dp/dt) simulated by the biomechanical model could provide a more stable estimate of this contractility estimate (with a better reproducibility), avoiding the differentiation of the measured data, which are often subjected to noise.For the functional indices derived from fully non-invasive data, the maximum and minimum aortic pressure will have to be replaced by a non-invasive arterial pressure measurement transferred to the central pressure (e.g. by using some modeling techniques11). In our proof-of-concept work we did not want to introduce additional bias into the model of ventricular mechanics simulating the P-V loop.
The CMR exam did not show any signs of a significantly increased filling pressure. A sensitivity analysis for the imposed 10mmHg filling pressure remains to be done. Surrogate methods estimating the level of filling pressure (typically using atrioventricular flow profile) and classifying the value to normal, increased and very high will need to be included when considering patients with failing ventricle. No aortic valve pathology was observed in our patients. The universality of our modeling framework allows to incorporate insufficient or stenosed valves10,12 and the aortic flow profile would be used in such a case.
Finally, only a limited number of patients with and only with rTOF were included in this study. They had no signs of LV failure. However, more heterogeneous groups and larger cohorts will need to be evaluated, prior to a routine clinical deployment.
Conclusion
Biomechanical modeling has the potential to substantially increase the possibilities of CMR and provide non-invasively important quantities otherwise accessible only through an invasive approach.Acknowledgements
The study was supported by the Inria-UTSW Associated Team TOFMOD. In addition, we would like to acknowledge Dr. Philippe Moireau, Inria research team MΞDISIM, for the development of the cardiac simulation software CardiacLab used in this work.References
1. Suga H et al. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circulation research. 1973; 32(3):314–322.
2. Casas B et al. Bridging the gap between measurements and modelling: a cardiovascular functional avatar. Scientific reports. 2017; 7(1):6214.
3. Seemann F et al. Noninvasive Quantification of Pressure-Volume Loops From Brachial Pressure and Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2019; 12(1):e008493.
4. Ruijsink B et al. Dobutamine stress testing in patients with Fontan circulation augmented by biomechanical modeling. PloS one. 2020; 15(2): e0229015.
5. Le Gall A et al. Monitoring of cardiovascular physiology augmented by a patient-specific biomechanical model during general anesthesia. A proof of concept study. PLOS ONE. 2020;15(5).
6. Caruel M et al. Dimensional reductions of a cardiac model for effective validation and calibration. Biomech Model Mechanobiol. 2014;13(4):897-914.
7. Chapelle D et al. An energy-preserving muscle tissue model: formulation and compatible discretizations. Int J Multiscale Comput Eng. 2012;10(2):189.
8. Castellanos DA et al. Left ventricular torsion obtained using equilibrated warping in patients with repaired Tetralogy of Fallot. Pediatric Cardiology. 2021; 42(6):1275-1283.
9. Gusseva M et al. Model-assisted time-synchronization of cardiac MR image and catheter pressure data, Proc. of Functional Imaging and Modeling of Heart FIMH 2021, Volume 12738 of Lecture Notes in Computer Science (LNCS), 2021; 362-372.
10. Gusseva M et al. Biomechanical Modeling to Inform Pulmonary Valve Replacement in Tetralogy of Fallot Patients after Complete Repair. Canadian Journal of Cardiology. 2021. https://doi.org/10.1016/j.cjca.2021.06.018
11. Muller LO et al. Reduced-Order Unscented Kalman Filter With Observations in the Frequency Domain: Application to Computational Hemodynamics. IEEE Transactions on Biomedical Engineering. 2018; 66(5):1269–1276.
12. Fučík R et al. Investigation of phase contrast magnetic resonance imaging underestimation of turbulent flow through the aortic valve phantom: Experimental and computational study by using lattice Boltzmann method. MAGMA. 2020; 33(5):649-662.
Figures