1307
Assessment of myocardium native T1 and perfusion using exercise CMR with a novel MRI-compatible supine ergometer1Department of Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
This study investigated the feasibility of a novel compact MRI-compatible ergometer to evaluate myocardial tissue characteristics and blood flow. The results showed a significant increase in heart rate, RPP, native T1 and MBF by using this ergometer in healthy controls. Our study has shown an excellent reproducibility in measuring free-breathing native myocardial T1, MBF and MPR during exercise. The pilot testing demonstrated that the novel compact MRI-compatible ergometer was successful at inducing a cardiac stress state and can able to characterise exercise physiology at every stage allowing high quality MR imaging during the stress.
Background:
Exercise imaging is known to have a higher sensitivity than pharmacological stressors to detect subclinical cardiac diseases (1, 2). This study aimed to show a new custom-made MRI-compatible ergometer and assess the reproducibility of measurements for myocardial native T1 and myocardial blood flow (MBF) at rest and exercise.Methods:
Ten healthy volunteers (5 females) underwent CMR scanning twice in a 3 T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). The whole imaging protocol is shown in Figure 1a. Native T1 maps at mid-ventricular short-axis locations were acquired by utilizing a modified Look-Locker inversion recovery (MOLLI) sequence with motion correction and a 5b(3b)3b scan scheme (3). Stress and rest perfusion images were acquired with a time interval of 15 min in between. The myocardial native T1 and MBF measurements were performed on the mid-ventricular short-axis slice positions. The native T1 maps were acquired at 1st and 3rd minute after the start of exercise; and MBF was acquired at 4th minute. The subjects alternately depressed pedals for 4 minutes at 60 Hz within the MR cavity. The MRI-compatible ergometer was set on the patient table as shown in Figure 1b. The reproducibility of the two tests was determined by the intra-group correlation coefficient (ICC) and coefficient of variation (CoV). The consistency of tests was analyzed by Bland-Altman method.Results:
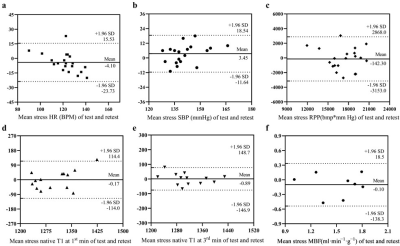
The mean exercise intensity was 25 W, with an exercise duration of 5 minutes. Exercise induced a 27% increase in systolic blood pressure and an increase of 74% and 65% in heart rate at the 1st and 3rd minutes. The rate pressure product increased 121% and 108% at the 1st and 3rd minutes (all p < 0.001). The exercise native T1 values at 1st and 3rd minutes were 1302 ± 64 ms and 1315± 61 ms, respectively, which were significantly larger than resting T1 (p<0.05). The effect of exercise stress from test and retest was shown in Figure 2. It was demonstrated that exercise T1 value at the 1st and 3rd minutes had strong reliability (ICC=0.75 and 0.89) and excellent reproducibility (CoV = 3.0% and 2.0%). Mean global rest MBF, exercise MBF and MPR were 0.9 ± 0.1 ml/min/100g, 1.6 ± 0.3 ml/min/100g, and 1.8 ± 0.2, respectively. Test-retest reliability of stress MBF was moderate [CoV 10.7%, ICC 0.84 (0.28;0.96)]. Test-retest reliability of MPR was good [ICC, 0.92 (0.68;0.98)]. The test–retest reproducibility is shown in Table 1 and Figure 3. Female subjects had higher positive changes in native T1 and MBF, compared to male.Table 1 Reproducibility of Rest and Stress Native T1 and Myocardial Blood Flows
| Parameter | Test 1 | Test 2 | CoV (%) | ICC (95% CI) | P value |
| Global T1 | |||||
| Rest | 1248 (34) | 1266 (42) | 1.68 | 0.87 (0.57; 0.96) | 0.001 ** |
| Stress 1 min | 1302 (76) | 1302 (51) | 3.05 | 0.75 (0.22; 0.92) | 0.009 ** |
| Stress 3 min | 1316 (57) | 1314 (67) | 2.02 | 0.89 (0.64; 0.97) | 0.001 ** |
| Recovery | 1057(50) | 1042 (27) | 2.84 | 0.62(-0.67; 0.92) | 0.095 |
| Global perfusion | |||||
| Rest MBF | 0.96 (0.29) | 0.96 (0.20) | 15.13 | 0.77 (-0.02; 0.95) | 0.027 * |
| Stress MBF | 1.49 (0.30) | 1.59 (0.30) | 10.70 | 0.84 (0.28; 0.96) | 0.010 * |
| MPR | 1.69 (0.45) | 1.72 (0.41) | 8.80 | 0.92 (0.68; 0.98) | 0.001 ** |
Discussion:
In the present study, we experimented on and investigated stress CMR by using a novel compact MRI-compatible ergometer and found the heart rate, systolic blood pressure and RPP increased significantly at 1st and 3rd min during the exercise stress. We have also observed that with exercise stress both myocardial native T1 and MBF increased. The pilot testing demonstrated that our device was successful at inducing a cardiac stress state, without the use of pharmaceuticals, while simultaneously allowing high quality MR imaging during the exercise stress. Furthermore, we have demonstrated the excellent inter-observer and scan-rescan reproducibility of native T1. It is feasible to assess myocardial tissue characteristics and blood flow by using the novel compact MRI-compatible ergometer during continuous in-scanner supine pedal exercise with free-breathing.Exercise has been recognized as the most important efficient physiological stimulus that increases the demand on myocardial oxygen. Consumption of myocardial oxygen is determined primarily by intramyocardial wall stress (i.e. the product of LV pressure and volume divided by LV wall thickness), contractility and heart rate (4,5). The increase in heart rate obtained in our study (74%) is in line with the results reported by the above studies of pharmacological stress, indicating that the exercise protocol of this study can be used to perform near real-time evaluation of the biventricular function and the left ventricular (LV) wall motion under physical stress and is better than that in a previous evaluation of dobutamine-associated analyses (6,7).
Conclusions:
Excellent reproducibility was shown in the assessment of native myocardial T1, MBF and MPR using our new custom-made MRI-compatible ergometer. This ergometer in the MR scanner has a great potential for future clinical application to accurately and noninvasively assess cardiovascular function under stress. Lastly, further studies are to be carried out to examine its clinical utility and feasibility in a variety of cardiovascular diseases.Acknowledgements
We appreciate Xiaoyue Zhou‘s assistance with the linguistic editing and proofreading of this paper. We also thank Ruoyang Li and Haichen Li for their help with acquiring the data and carrying out the experiments. This work was supported by the Radiology, Medical Imaging of West China Hospital,Sichuan University, Chengdu, Sichuan, China.References
1. Arnold JR, McCann GP. Cardiovascular magnetic resonance: applications and practical considerations for the general cardiologist. Heart 2020;106(3):174-181.
2. Lee SE, Nguyen C, Xie Y, et al. Recent Advances in Cardiac Magnetic Resonance Imaging. Korean Circ J 2019;49(2):146-159.
3. Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 2012;14(1):63.
4. Foster EL, Arnold JW, Jekic M, et al. MR-compatible treadmill for exercise stress cardiac magnetic resonance imaging. Magn Reson Med 2012;67(3):880-889.
5. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 2008;88(3):1009-1086.
6. Steding-Ehrenborg K, Jablonowski R, Arvidsson PM, Carlsson M, Saltin B, Arheden H. Moderate intensity supine exercise causes decreased cardiac volumes and increased outer volume variations: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2013;15(1):96.
7. Vasu S, Little WC, Morgan TM, et al. Mechanism of decreased sensitivity of dobutamine associated left ventricular wall motion analyses for appreciating inducible ischemia in older adults. J Cardiovasc Magn Reson 2015;17(1):26.
Figures