1302
Dynamic BOLD MRI Shows Greater Foot Ischemia and Blunted Reperfusion Following Cuff-Occlusion Challenge in Diabetic Patients with Foot Ulcers
Scott J. Edwards1, Jingting Yao2, Marcos Coutinho Schechter1, Maya Fayfman1, Gabriel Santamarina1, Paula Nesbeth1, Vincent Giacalone1, Gerado Blanco1, Rabindra Tirouvanziam1, Jessica Alvarez1, Benjamin Risk1, Ravi Rajani1, and David A. Reiter1
1Emory University, Atlanta, GA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States
1Emory University, Atlanta, GA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Diabetic foot ulcer (DFU) patients often face incomplete wound healing which leads to a long-term state of inflammation and risk of infection. Prolonged wound healing is thought to result in part from impaired tissue perfusion due to microvascular disease. This study applies dynamic blood-oxygenation-level-dependent (BOLD) imaging to quantify reperfusion of tissue after induced occlusion and ischemia in the foot. When compared to a healthy population, DFU patients show an increased ischemic state during cuff occlusion and decreased reperfusion after release. Quantification of cuff-occlusion parameters may assist in the classification of DFUs and be used to predict the prognosis and enhance treatment plan.
Introduction
Diabetic foot disease is the leading cause of preventable limb loss in the United States [1]. Specifically, diabetic foot ulcers (DFUs) require resource-intensive and expensive care that can leave up to 25% of ulcers to fail to heal [2, 3]. Early detection and stratification of the severity of DFUs is clinically important to determine effective treatment plans. Strong clinical evidence demonstrates both initiation of ulcers and poor wound healing are related to reduced tissue perfusion and ischemia [2, 4]. Existing non-invasive tissue perfusion assessments, such as the ankle-brachial index, have poor predictive value for wound healing [5] and poor correlation to tissue perfusion in DFUs when measured by MRI approaches [6]. MRI protocols developed for studying microvascular volume and reserve in aging muscle [7-10] have been adapted to evaluate tissue perfusion in the setting of DFU. This study seeks to quantify T2* signals during tissue perfusion and how they differ in a diabetic and healthy population. The patients with DFU are expected to exhibit abnormalities in the T2* signal compared to a healthy population during a vasculature occlusion and reperfusion protocol.Methods
BOLD MRI scans were acquired from the feet of five healthy subjects (2 female) and five patients with type 2 diabetes (2 female) with plantar forefoot ulcers using a 3 Tesla Prisma Fit (Siemens MAGNETOM Prisma Fit, Siemens Healthcare, Erlangen, Germany MAGNETOM) (Table 1). Selection criteria for DFU patients consisted of diagnosis of type II diabetes with the presence of unhealed plantar ulcer for at least one month. Patients were excluded if there was evidence of active osteomyelitis, indication of macrovascular disease or chronic kidney disease greater than stage 3. All imaging was performed in the early morning after an overnight fast.Dynamic BOLD imaging with coverage of the entire foot was achieved using a dual-echo gradient-echo sequence with a single-shot echo planar readout with 700 dynamics, TR = 1000 ms, TE1 = 15 ms, and TE2 = 40.34 ms. A rapidly inflating pneumatic cuff was placed around the ankle of each subject to occlude arterial blood supply to the foot. BOLD MRI measurements were made dynamically during the cuff procedure. The ischemia-reperfusion protocol consisted of 100 seconds of baseline measurements with the cuff deflated, followed by 300 seconds of ischemia with the cuff inflated to 50mmHg above systolic blood pressure, followed by 300 seconds of recovery with the cuff deflated. The toes, heel, medial plantar, lateral plantar, and dorsal angiosomes were generated using the calcaneus, talus, and metatarsals as landmarks [11] and are shown in Figure 1. The VOIs used in this analysis are the lateral and medial plantar angiosomes. Each series of dynamics was inspected for motion and artifacts that could impact analysis before continuing.
The two echoes were fit to an exponential decay model to estimate average values of tissue T2* over the respective VOI.. Baseline values reflect the average signal over the first 100 seconds. The time-series of T2* signals were normalized to the respective baseline values. Minimal ischemic value is defined as minimum normalized T2* value during cuff occlusion. Peak reperfusion value is defined as the maximum normalized T2* value after the cuff was released. Time to minimum ischemic value and time to peak reperfusion value is the amount of time from onset to the respective values. Statistical differences were calculated with two-sample t-tests (alpha = 0.05) and confirmed with Wilcoxon rank-sum tests. All data analysis was implemented using MATLAB-based toolboxes.
Results and Discussion
DFU patients and healthy controls showed substantially different BOLD responses to the cuff occlusion protocol. Table 2 outlines the results of the five parameters. The starting baseline values of both groups were similar. During the occlusion stage, the signals of patients with DFUs dropped significantly lower in the medial plantar regions. There was no difference in the time it took to reach the minimal ischemic value in either region, some subjects reached a minimal value and plateaued while other subjects never plateaued. In both the lateral and medial plantar regions, the DFU patients had a significantly lower maximal reperfusion value and longer time-to-peak than the healthy controls. Additionally, the DFU group barely reached baseline values during reperfusion while the healthy controls overshot baseline. Our results indicate blunted perfusion characteristics in the patients with DFUs. Figures 2 and 3 show the average traces for each population in the medial and lateral plantar regions.Conclusion
The aim of this study was to use BOLD imaging to find quantifiable parameters to improve categorization and stratification of DFUs for future studies. Higher quality metrics will inform decisions regarding treatment plans and outcomes. This analysis shows there are significant differences in how the DFU feet react to an ischemia/reperfusion cuff protocol compared to controls. Future work will address the same parameters in the last three angiosomes as well as increase predictive power by combining these results with other modalities of imaging.Acknowledgements
Financial support for this work was provided by the NIDDK Diabetic Complications Consortium (RRID:SCR_001415, www.diacomp.org), grants DK076169 and DK115255.References
1. Promotion, N.C.f.C.D.P.a.H., National Diabetes Statistics Report, 2017 Estimates of Diabetes and Its Burden in the United States, D.o.D. Translation, Editor.2. Prompers, L., et al., Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia, 2008. 51(5): p. 747-55.
3. Ndosi, M., et al., Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med, 2018. 35(1): p. 78-88.
4. Falanga, V., Wound healing and its impairment in the diabetic foot. Lancet, 2005. 366(9498): p. 1736-43.
5. Brownrigg, J.R., et al., Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev, 2016. 32 Suppl 1: p. 128-35.
6. Edalati, M., et al., Intravenous contrast-free standardized exercise perfusion imaging in diabetic feet with ulcers. J Magn Reson Imaging, 2018.
7. Adelnia, F., et al., The Role of Muscle Perfusion in the Age-Associated Decline of Mitochondrial Function in Healthy Individuals. Front Physiol, 2019. 10: p. 427.
8. Reiter, D.A., et al. Modeling skeletal muscle perfusion through application of the continuous time random walk model to diffusion-weighted images. in International Society for Magnetic Resonance in Medicine. 2018. Paris France.
9. Cameron, D., et al. Diffusion-weighted triple-fat-suppressed echo-planar imaging provides 'anomalous' diffusion metrics for assessment of muscle quality in the human thigh. in International Society of Magnetic Resonance in Medicine 2015 23rd Scientific Meeting and Exhibition. 2015. Toronto, Canada.
10. Cameron, D., et al., The effect of noise and lipid signals on determination of Gaussian and non-Gaussian diffusion parameters in skeletal muscle. NMR Biomed, 2017. 30(7).
11. Stacy, M.R., et al., Application of BOLD Magnetic Resonance Imaging for Evaluating Regional Volumetric Foot Tissue Oxygenation: A Feasibility Study in Healthy Volunteers. Eur J Vasc Endovasc Surg, 2016. 51(5): p. 743-9.
Figures
Table 1.
Subject Data and comparison
Table
2. Results of occlusion/reperfusion. Percentage
values are normalized to the baseline.
Figure
1. Plantar view of angiosome segmentation showing heel region (green), toes
region (grey), medial plantar (blue) and lateral plantar (red).

Figure
2. Average traces of the DFU and HC in the medial plantar region. Cuff
occlusion occurred at t = 100s and released at t = 400s. The thick traces
represent group average normalized T2* values and dotted traces represent
average +/- standard deviation.
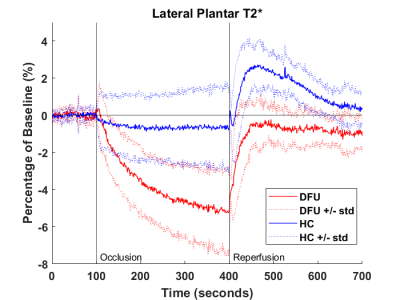
Figure 3. Average traces of the DFU and HC in the lateral plantar region. Cuff occlusion
occurred at t = 100s and released at t = 400s. The thick traces represent group
average normalized T2* values and dotted traces represent average +/- standard
deviation.
DOI: https://doi.org/10.58530/2022/1302