1298
Effects of Sleep on perivascular space in healthy population
Nien-Chu Shih1, Karen Lincoln2, Farshid Sepehrband1, and Jeiran Choupan1
1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Suzanne Dworak-Peck School of Social Work, University of Southern California, Los Angeles, CA, United States
1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Suzanne Dworak-Peck School of Social Work, University of Southern California, Los Angeles, CA, United States
Synopsis
The perivascular space (PVS) has been reported to clear Amyloid-β (Aβ) and metabolic wastes during sleep. However, the relationship between sleep and PVS in a healthy cohort is still unclear. We investigate the association between PVS and sleep across different age groups. We found that the effect of sleep on PVS volume varies in the young and older population.
Body of Abstract
Introduction: The perivascular space (PVS) has been reported to play an important role in the clearance of Amyloid-β (Aβ) and metabolic wastes during sleep 1,2. Enlarged PVS can be detected in magnetic resonance imaging (MRI) in disease cases 3,4. A body of research has investigated the relationship between PVS and sleep quality and found that PVS volume increases with sleep disruption 5–8. However, the relationship between sleep and PVS in a healthy cohort is still unclear. Therefore, we used magnetic resonance imaging data from the Human Connectome Project Aging (HCPA) 9 dataset to investigate the relationship between sleep and PVS in different age groups in a healthy population.Methods: Data of 555 healthy subjects were utilized from HCPA dataset. We quantified PVS volume fraction in centrum semiovale (CSO) and basal ganglia (BG) from the Enhanced PVS Contrast (EPC) image 10. We measured duration of sleep, sleep efficiency, and sleep quality by self-report with the Pittsburgh Sleep Quality Index (PSQI) questionnaire, and used multiple linear regression to analyze the data, correcting for age, sex, race, and BMI.
Results: Using age stratified subgroups, we found that there is larger CSO-PVS volume fraction in younger participants (36-54 years old) who had less sleep time. Our analysis showed that sleeping 1 hour less, will lead to an increase in CSO-PVS by 0.096 units. Another interesting finding was that older participants (65+ years old) who had better sleep efficiency and better sleep quality had larger BG-PVS volume fraction. In addition, this relationship did not differ by BMI status in the older population. Finally, there was no significant relationship between sleep and PVS in the middle-age group (55-65 years old).
We further investigated the effect of sleep on PVS in African American participants (compared to other races) available in HCPA aging dataset. Compared to other races, the effect of sleep on CSO-PVS volume fraction for younger African Americans (N=56) was not significant compared to other races. However, the sleep quality and sleep efficiency significantly affected BG-PVS volume fraction in older African Americans.
Discussion: Among younger participants, lower sleep duration was associated with increased CSO-PVS volume fraction. In contrast, healthy older participants who had better sleep efficiency and sleep quality had larger BG-PVS volume fraction. Our results are contrary to previous PVS studies which mentioned that poor sleep is associated with enlarged PVS in disease cohort 8,11,12. One possibility could be that in comparison to findings from previous works, we studied a healthy cohort. The enlarged PVS for a healthy population may have different mechanisms to clear brain metabolic wastes than an unhealthy population. In addition, our results showed that the effect of sleep on PVS volume was different for younger adults compared to older adults. We also found that the effects of sleep on PVS volume fraction differed by race. This is the first study to report an association between PVS volume fraction and sleep in a racially/ethnically diverse sample. This study provides more generalizability and a different view of the association between sleep and PVS in healthy cohorts across different age and racial groups.
Conclusion: We investigated the relationship between PVS volume and sleep measurements in an aging healthy cohort using data from HCP-Aging. The effect of sleep on PVS volume varied in young and older populations.
Acknowledgements
This project is supported by the National Institutes of Health: RF1MH123223-01.References
1. Reddy OC, van der Werf YD. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sciences 2020; 10: 1–16.2. Shokri-Kojori E, Wang G-J, Wiers CE, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 2018; 115: 4483–4488.
3. Ramirez J, Berezuk C, McNeely AA, et al. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook dementia study. J Alzheimer’s Dis 2015; 43: 415–424.
4. Potter GM, Chappell FM, Morris Z, et al. Cerebral perivascular spaces visible on magnetic resonance imaging: Development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis 2015; 39: 224–231.
5. Charidimou A, Martinez-Ramirez S, Viswanathan A. Waking up MRI-visible perivascular spaces and drainage research. Sleep 2015; 38: 845–846.
6. Lysen TS, Yilmaz P, Dubost F, et al. Sleep and perivascular spaces in the middle-aged and elderly population. J Sleep Res 2021; 1–9.
7. Aribisala BS, Riha RL, Valdes Hernandez M, et al. Sleep and brain morphological changes in the eighth decade of life. Sleep Med 2020; 65: 152–158.
8. Ramirez J, Holmes MF, Berezuk C, et al. MRI-visible perivascular space volumes, sleep duration and daytime dysfunction in adults with cerebrovascular disease. Sleep Med 2021; 83: 83–88.
9. Bookheimer SY, Salat DH, Terpstra M, et al. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 2019; 185: 335–348.
10. Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep 2019; 9: 1–12.
11. Berezuk C, Ramirez J, Gao F, et al. Virchow-Robin spaces: Correlations with polysomnography-derived sleep parameters. Sleep 2015; 38: 853–858.
12. Opel RA, Christy A, Boespflug EL, et al. Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J Cereb Blood Flow Metab 2019; 39: 2258–2267.
Figures
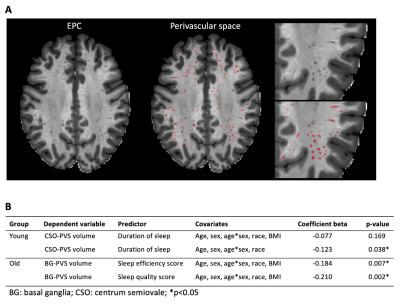
The overview of this study. A. Enhanced PVS Contrast (EPC) image overlaps with Perivascular space (PVS) mask (red area). B. The table of the association between PVS volume and sleep. We applied linear regression model to investigate the relationship between PVS volume and the sleep measurements in young and old group respectively. In young population, CSO-PVS volume is negatively associated with duration of sleep. On the other hand, BG-PVS volume is negatively associated with sleep quality score and sleep efficiency score. Significant *p<0.05
DOI: https://doi.org/10.58530/2022/1298